Figures & data
Figure 1. Effect of cholesterol depletion on detergent insolubility of 5-HT1AR-EYFP. (A) CHO-5-HT1AR-EYFP cells under normal and cholesterol depleted conditions are shown before and after treatment with 0.05% (w/v) cold Triton X-100 respectively (reproduced from Citation[17]). Cholesterol depletion was performed by treating the cells with 5 mM MβCD. Images represent combined mid-plane confocal sections of the same group of cells before and after detergent extraction. Fluorescence intensity of the same group of cells before and after detergent treatment was quantitated using the Meridian DASY Master program, and detergent insolubility of the 5-HT1A receptor was assessed by determining the residual fluorescence. Scale bar represents 10 µm. See Materials and methods for other details. (B) Frequency distribution profiles of detergent insolubility of 5-HT1AR-EYFP determined under normal and cholesterol-depleted conditions. Detergent insolubility of 5-HT1AR-EYFP was estimated by measuring the residual fluorescence following detergent extraction as described in Materials and methods. The plots shown represent 28 different data points of residual fluorescence measurements each for the normal and cholesterol-depleted conditions. The frequency of occurrence of various values has been normalized to the total number of measurements. See Materials and methods for other details.
![Figure 1. Effect of cholesterol depletion on detergent insolubility of 5-HT1AR-EYFP. (A) CHO-5-HT1AR-EYFP cells under normal and cholesterol depleted conditions are shown before and after treatment with 0.05% (w/v) cold Triton X-100 respectively (reproduced from Citation[17]). Cholesterol depletion was performed by treating the cells with 5 mM MβCD. Images represent combined mid-plane confocal sections of the same group of cells before and after detergent extraction. Fluorescence intensity of the same group of cells before and after detergent treatment was quantitated using the Meridian DASY Master program, and detergent insolubility of the 5-HT1A receptor was assessed by determining the residual fluorescence. Scale bar represents 10 µm. See Materials and methods for other details. (B) Frequency distribution profiles of detergent insolubility of 5-HT1AR-EYFP determined under normal and cholesterol-depleted conditions. Detergent insolubility of 5-HT1AR-EYFP was estimated by measuring the residual fluorescence following detergent extraction as described in Materials and methods. The plots shown represent 28 different data points of residual fluorescence measurements each for the normal and cholesterol-depleted conditions. The frequency of occurrence of various values has been normalized to the total number of measurements. See Materials and methods for other details.](/cms/asset/7b907fd7-e8c6-46f8-b439-981c5a4c3a23/imbc_a_142156_f0001_b.jpg)
Table I. Detergent insolubility of 5-HT1AR-EYFP at various concentrations of Triton X-100a.
Figure 2. Effect of treatment of CHO-5-HT1AR-EYFP cells in culture with 5 mM MβCD on the lipid composition of membranes. Cholesterol (light gray bars) and phospholipid (dark gray bars) contents were assayed as described in Materials and methods. The lipid composition of control membranes isolated from CHO-5-HT1AR-EYFP cells, which have not been treated with MβCD, is shown for comparison. Values have been normalized with respect to the total protein content. Data represent the means±SE of at least three independent experiments. See Materials and methods for other details.
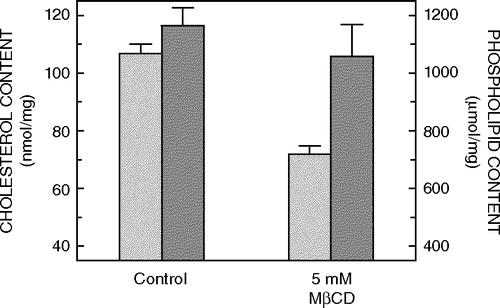
Figure 3. Detergent insolubility of 5-HT1AR-EYFP upon stimulation by 10 µM serotonin. (A) CHO-5-HT1AR-EYFP cells were incubated with the endogenous ligand 5-HT, followed by treatment with 0.05% (w/v) cold Triton X-100. Cells were imaged as described in Materials and methods. The images represent combined mid-plane confocal sections of the same group of cells before and after detergent extraction. Scale bar represents 10 µm. See Materials and methods for other details. (B) Frequency distribution profiles of detergent insolubility of 5-HT1AR-EYFP under normal and ligand stimulation (+5-HT) conditions are shown. Detergent insolubility of 5-HT1AR-EYFP was estimated by measuring the residual fluorescence following detergent extraction as described in Materials and methods. The plots shown represent 14 data points of residual fluorescence measurements in the case of serotonin stimulation, and 28 data points in the case of unstimulated (normal) cells. The frequency of occurrence of various values has been normalized to the total number of measurements made. See Materials and methods for other details.
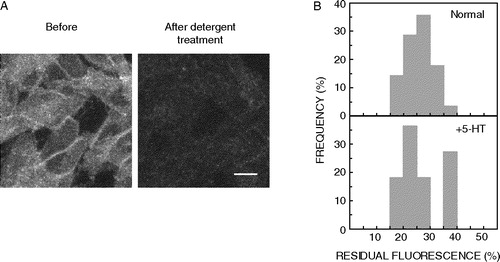
Figure 4. Schematic representation of possible membrane reorganization (shown as top view) induced by cholesterol depletion and its implications on detergent insolubility of 5-HT1AR-EYFP in membranes. Membrane organization under normal (A) and cholesterol-depleted (B) conditions is shown. The EYFP-tagged serotonin1A receptor (5-HT1AR-EYFP) is shown as filled squares. Current understanding of membrane organization involves heterogeneities (domains/rafts) on the cell surface (shown as circles), with boundaries depicted as being discontinuous due to the tendency of cholesterol to form intermixed domains Citation[7] (shown in panel A). As a result of this, while the presence of such domains could be inferred through the phenomenon of detergent resistance, they may not be detected as separate entities through light microscopy. Due to the role of cholesterol in domain miscibility, lowering its levels from membranes could lead to segregation and coalescence of microdomains into large micrometer-scale ordered domains Citation[33] (shown in panel B). The eventual distribution of 5-HT1AR-EYFP in such domains could determine the fraction of detergent insoluble receptor. Since our results suggest a small increase in detergent insoluble receptor upon cholesterol depletion, it could be speculated that a greater number of receptors may be included into the large micrometer-scale ordered domains (shown in panel B).
![Figure 4. Schematic representation of possible membrane reorganization (shown as top view) induced by cholesterol depletion and its implications on detergent insolubility of 5-HT1AR-EYFP in membranes. Membrane organization under normal (A) and cholesterol-depleted (B) conditions is shown. The EYFP-tagged serotonin1A receptor (5-HT1AR-EYFP) is shown as filled squares. Current understanding of membrane organization involves heterogeneities (domains/rafts) on the cell surface (shown as circles), with boundaries depicted as being discontinuous due to the tendency of cholesterol to form intermixed domains Citation[7] (shown in panel A). As a result of this, while the presence of such domains could be inferred through the phenomenon of detergent resistance, they may not be detected as separate entities through light microscopy. Due to the role of cholesterol in domain miscibility, lowering its levels from membranes could lead to segregation and coalescence of microdomains into large micrometer-scale ordered domains Citation[33] (shown in panel B). The eventual distribution of 5-HT1AR-EYFP in such domains could determine the fraction of detergent insoluble receptor. Since our results suggest a small increase in detergent insoluble receptor upon cholesterol depletion, it could be speculated that a greater number of receptors may be included into the large micrometer-scale ordered domains (shown in panel B).](/cms/asset/ddeb390b-a094-47cd-96e6-ceb3a94f03d3/imbc_a_142156_f0004_b.jpg)
