Figures & data
Table I. Sequences of selected antibiotic helical peptides. Residues that are positively charged at pH 7 or pH 5 are highlighted in bold whilst negatively charged residues are marked in grey. *Moronecidin is also known as piscidin 2.
Figure 1. Intrinsic tryptophan fluorescence spectra of Trp2 of pleurocidin in the presence of liposomes of varying lipid composition at neutral and acidic pH are shown (A). The presence of PG causes a slight blue shift and increase in fluorescence intensity irrespective of the zwitterionic component of the liposomes whilst the spectra obtained at acidic pH are of reduced intensity. A Stern-Vollmer plot of the effect of adding an aqueous acrylamide quencher on tryptophan fluorescence intensity (B) reveals the depth of pleurocidin penetration into the model membranes.
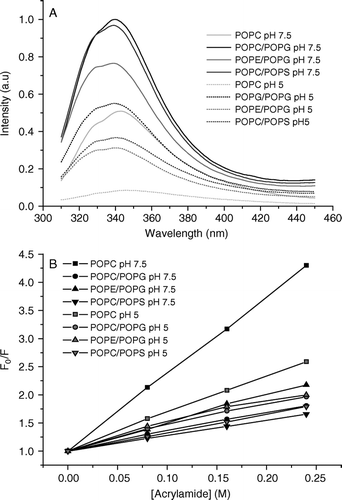
Figure 2. 2H echo spectra of POPE-d31 or POPG-d31 in lipid vesicles. Vesicles contained POPE/POPE-d31/POPG (2:1:1) (A) or POPE/POPG-d31 (3:1) (B) in the absence (grey lines) or presence (black lines) of 2.5 mole% pleurocidin at pH 7.5. Spectra were recorded on a Bruker Avance 300 spectrometer at 298K. 2H order parameter profiles for the same peptide containing vesicles at pH 7.5 and also with an elevated pleurocidin concentration of 3.3 mole% are shown calculated relative to peptide free vesicles (C).
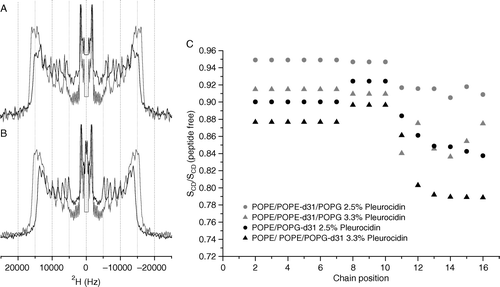
Figure 3. 2H echo spectra of POPE-d31 or POPG-d31 in lipid vesicles containing POPE/POPE-d31/POPG (2:1:1) (A) or POPE/POPG-d31 (3:1) (B) and 2.5 mole% pleurocidin at pH 7.5 (grey line) and pH 5 (black line). The change in pH has very little effect on the spectra. Spectra were recorded on a Bruker Avance 300 spectrometer at 298K.
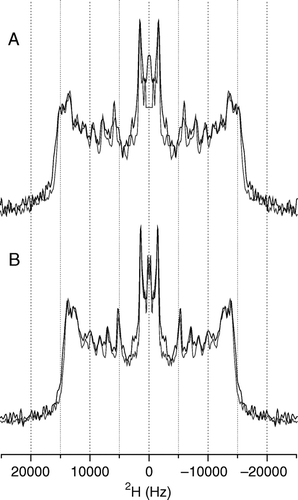
Figure 4. The static 15N NMR spectrum of 15N-alanine10, alanine19 and leucine21 labelled pleurocidin reconstituted in POPE/POPG (3:1) bilayers and deposited on glass plates oriented perpendicular to B0 (A). Spectra were acquired for samples prepared at both pH 7 (black line) and pH 5 (grey line). The observed chemical shift is characteristic of a peptide oriented parallel to the membrane surface. 31P echo spectra of POPE/POPG (3:1) bilayers (B), prepared in the same way at pH 7, in the absence (top) and presence (bottom) of 2.5 mole% pleurocidin.
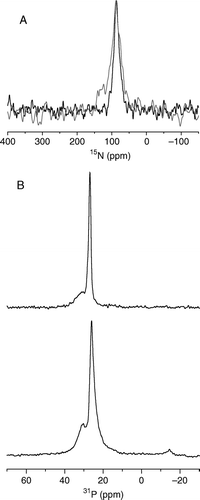
Figure 5. Structure of Pleurocidin based on the coordinates 1Z64.pdb obtained from the protein databank Citation[1] with the cationic and intrinsic fluorescing residues shown. A side view (A) reveals the distribution of the lysine and histidine residues along one face of the helix and the relative orientation of the tryptophan and tyrosine side chains to the helix. A view along the helix (B) shows that all three histidine residues are oriented along the spine of the helix and would interact only weakly with the lipid head groups of anionic lipids when oriented in this manner. This Figure is reproduced in colour in Molecular Membrane Biology online.
![Figure 5. Structure of Pleurocidin based on the coordinates 1Z64.pdb obtained from the protein databank Citation[1] with the cationic and intrinsic fluorescing residues shown. A side view (A) reveals the distribution of the lysine and histidine residues along one face of the helix and the relative orientation of the tryptophan and tyrosine side chains to the helix. A view along the helix (B) shows that all three histidine residues are oriented along the spine of the helix and would interact only weakly with the lipid head groups of anionic lipids when oriented in this manner. This Figure is reproduced in colour in Molecular Membrane Biology online.](/cms/asset/d186911d-dec0-4793-90c6-af1125c16e11/imbc_a_148513_f0005_b.jpg)