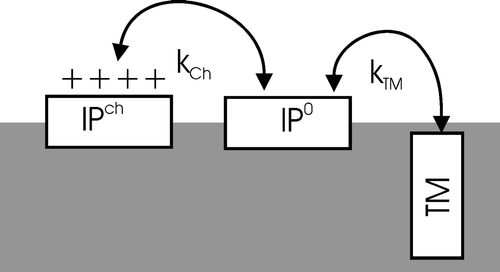
Figures & data
Figure 1. Sequence and helical wheel diagram of LAH4X4 peptides. Whereas the hydrophobic side B consists of leucines and alanines, face A is assembled from the amino acids X and flanked by two histidines on each side.
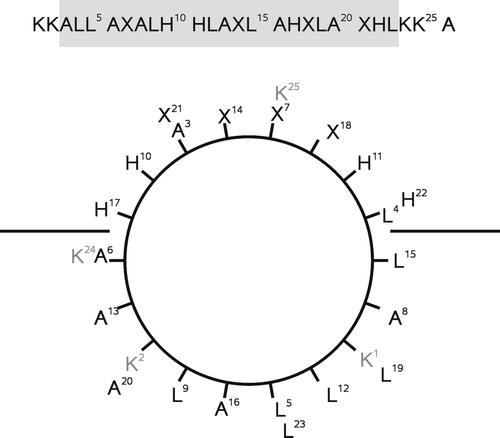
Figure 3. The fraction of transmembrane aligned peptide, pTM, as a function of pH at different transfer energies ΔG/RT. The titration curves were simulated with pKa values 6.0 for the histidine side chains. The simulations shown represent the following values of ΔG/RT: -8 (solid line), -2 (hatched line), 0 (dotted line), and 2 (hatched-dotted line).
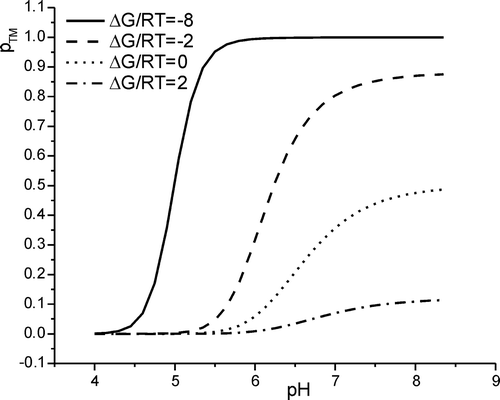
Figure 4. The calculated shifts for the in-plane to transmembrane transition pH as compared to the pKa value common to all histidines is shown as a function of changes in hydrophobicity of the X-amino acids, ΔG/RT. Whereas the solid line corresponds to the pH where half the maximum of pTM is reached, the dotted line indicates the pH value where half the peptides are in a transmembrane state, pTM=50%. The dashed line represents the linear approximation obtained for very hydrophobic peptides (large negative ΔG). See text for details.
Figure 5. The amide region of ATR-FTIR spectra of LAH4F4 reconstituted into oriented POPC membranes is shown at pH 8 (A) and at pH 3 (B). The incident light was polarized in a parallel (dotted lines) or in a perpendicular orientation (hatched lines). The difference spectra (solid lines) are obtained by subtracting the perpendicular polarized spectrum from the parallel polarized spectrum. For this Figure the latter was multiplied by a factor 1.35 before subtraction, which corrects for the difference of strength of the evanescent power for the parallel and perpendicular polarisations as discussed in detail in reference Citation[17].
![Figure 5. The amide region of ATR-FTIR spectra of LAH4F4 reconstituted into oriented POPC membranes is shown at pH 8 (A) and at pH 3 (B). The incident light was polarized in a parallel (dotted lines) or in a perpendicular orientation (hatched lines). The difference spectra (solid lines) are obtained by subtracting the perpendicular polarized spectrum from the parallel polarized spectrum. For this Figure the latter was multiplied by a factor 1.35 before subtraction, which corrects for the difference of strength of the evanescent power for the parallel and perpendicular polarisations as discussed in detail in reference Citation[17].](/cms/asset/3b153f25-1433-4ec9-8e77-90cf701ee322/imbc_a_173837_f0005_b.gif)
Figure 6. CD spectra of 10 µM LAH4L4 in the presence of 1000 µM small unilamellar POPC vesicles at pH 4.0 (dotted line) pH 5.0 (dash-dot line), pH 6.0 (dashed line) and pH 7 (solid line)
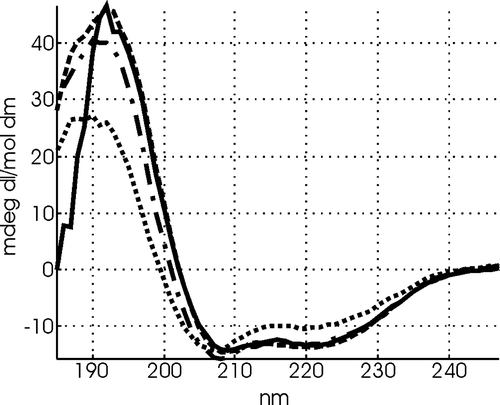
Figure 7. The dichroic ratio RATR of LAH4X4 peptides reconstituted into oriented POPC phospholipids bilayers are shown as a function of pH. A. LAH4F4, B. LAH4L4, C. LAH4V4, D. LAH4W4, E. LAH4I4., F. LAH4A3L. The experiments were performed in duplicate.
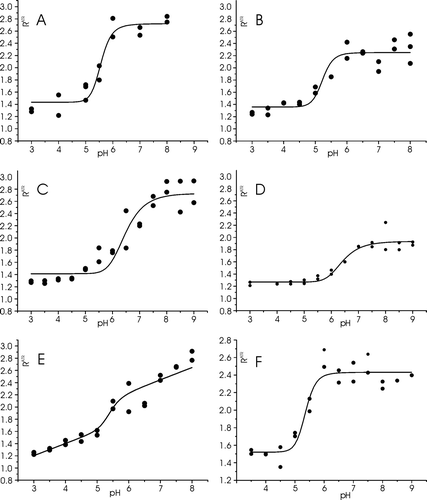
Table I. Analysis of the pH-dependent dichroic ratio of LAH4X4 peptides as observed in oriented ATR-FTIR experiments. A fit of the experimental data was obtained using Equations 11 and 12.
Figure 8. Summary of the relative contributions of the amino acid X to ΔG of transfer from in-plane to transmembrane alignments (cf. text for details). The values are scaled to take into account the influence of one X amino acid side chain within the peptide. The LAH4L4 peptide is taken as a reference. The boxes indicate the experimental errors obtained during the line fit analyis of ΔG/RT (). The top scale indicates values obtained when calculating the difference ΔG(aqueous→octanol) - ΔG(aqueous→membraneinterface) as published in Citation[6].
![Figure 8. Summary of the relative contributions of the amino acid X to ΔG of transfer from in-plane to transmembrane alignments (cf. text for details). The values are scaled to take into account the influence of one X amino acid side chain within the peptide. The LAH4L4 peptide is taken as a reference. The boxes indicate the experimental errors obtained during the line fit analyis of ΔG/RT (Table I). The top scale indicates values obtained when calculating the difference ΔG(aqueous→octanol) - ΔG(aqueous→membraneinterface) as published in Citation[6].](/cms/asset/b6dd037f-89a5-4012-a9ac-7bf54b6fc906/imbc_a_173837_f0008_b.gif)