Figures & data
Figure 1. SDS-PAGE profile of Tshβ purification. The outer membranes were collected following the lysis of bacterial cells. Proteins were extracted from the outer membranes. Tshβ was purified from the extract. Fractions containing Tshβ were combined, dialysed, and concentrated. Protein samples were resolved on 12% SDS-PAGE followed by Coomassie-blue staining. Lanes: 1, outer membranes of XL1-Blue/pSWK30; 2, outer membranes of XL1-Blue/pTsh-ΔN2 (Tshβ); 3, protein extract from AW741/pTsh-ΔN2 (Tshβ) membranes, acetone precipitated; 4, purified Tshβ. Arrow indicates the position of Tshβ.
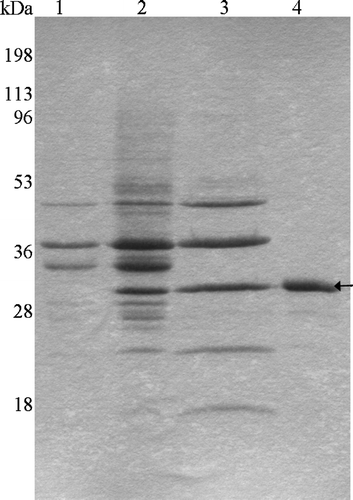
Figure 2. Heat modifiability of purified Tshβ. Purified Tshβ and outer membranes were heated for 5 min at either 100°C or 42°C in the presence of SDS loading buffer and then cooled down for 5 min on ice. Samples were loaded immediately and resolved on 12% SDS-PAGE. Protein bands were visualized by overnight Coomassie staining. Lanes: 1, outer membranes of XL1-Blue/pWSK30 heated to 42°C; 2, outer membranes of XL1-Blue/pTsh-ΔN2 heated to 42°C; 3, purified Tshβ heated to 42°C; 4, outer membranes of XL1-Blue/pWSK30 heated to 100°C; 5, outer membranes of XL1-Blue/pTsh-ΔN2 heated to 100°C; 6, purified Tshβ heated to 100°C. Oval arrow indicates the unfolded Tshβ and open arrow indicates the folded Tshβ.
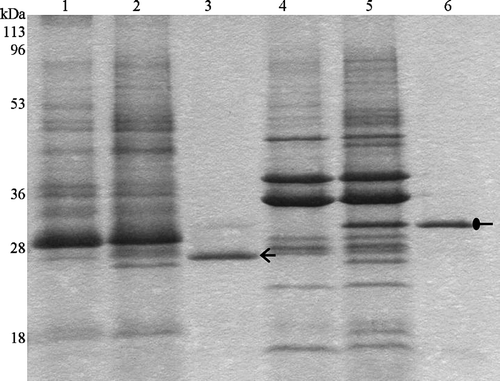
Figure 3. Trypsin digestion of Tshβ. Native and denatured outer membrane proteins and purified Tshβ were incubated with 30 µg/ml trypsin for 1 h at 37°C. Reactions were terminated by boiling for 10 min and addition of 10 mM PMSF. Fractions in lanes 5–7 were acetone precipitated overnight. All fractions were mixed with loading buffer, boiled for 5 min and resolved on 12% SDS-PAGE. Protein bands were visualized following the silver staining. Lanes: 1, outer membranes of XL1-Blue/pWSK30; 2, outer membranes of XL1-Blue/pTsh-ΔN2; 3, denatured outer membranes of XL1-Blue/pTsh-ΔN2 with 30 µg/ml trypsin; 4, outer membranes of XL1-Blue/pTsh-ΔN2 with 30 µg/ml trypsin; 5, Tshβ, the band at 25 kDa is unrelated to Tshβ or trypsin; 6, denatured Tshβ with 30 µg/ml trypsin; 7, folded Tshβ with 30 µg/ml trypsin. Arrow indicates position of Tshβ.
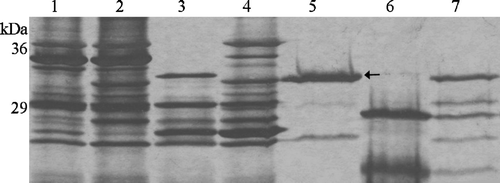
Figure 4. Profile of Tshβ and OmpC on BN-PAGE. Protein samples were incubated with loading buffer for 5 min on ice and resolved on 15–25% gradient gel. Lanes: 1, OmpC without detergent; 2, OmpC with 10 mM SB3-12, 3, Tshβ without detergent; 4, Tshβ with 10 mM SB3-12.
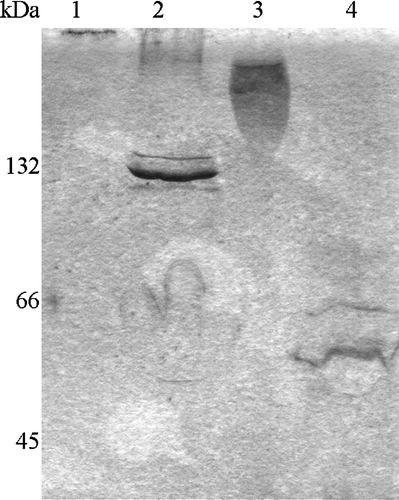
Figure 5. Cross-linking of Tshβ, Tshs, and OmpC. After purified proteins were cross-linked with DSS, reactions were terminated with 20 mM Tris-Cl (pH 7.5). Tshβ and OmpC were precipitated overnight with acetone. Protein samples were mixed with loading buffer, and after the boiling for 5 min, were resolved on SDS-PAGE (6% for Tshs and 10% for Tshβ and OmpC). Panel A: 1, Tshβ; 2, cross-linked Tshβ; Panel B: 1, cross-linked Tshs; 2, Tshs; Panel C: 1, OmpC; 2, cross-linked OmpC.
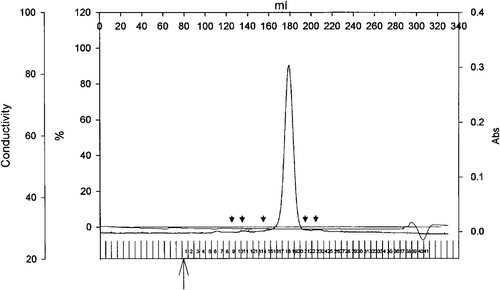
Figure 6. Elution profile of Tshs by size exclusion chromatography. Purified Tshs (6.4 mg) was loaded on a Superdex 200 26/60 column. The flow rate was 1 ml/min and protein presence was monitored at 280 nm. The arrowheads indicate elution volumes of standards. From left to right, standards included thyroglubulin (MW 669 kDa), ferritin (MW 440 kDa), catalase (MW 232 kDa), albumin (MW 67 kDa), ovalbumin (MW 43 kDa). Tshs eluted in a single peak that corresponds to an estimated molecular weight of 110 kDa.