Figures & data
Figure 1. Selective recognition of sphingomyelin by wt and W20A lysenin-His. Interaction of the proteins with various lipids was examined using a protein-lipid overlay assay. Indicated amounts of lipids were spotted onto nitrocellulose and exposed to 3 µg/ml of wt or W20A lysenin-His and anti-His IgG-peroxidase. Immunoreactive spots were revealed by chemiluminescence. DOPC, dioleoyl phosphatidylcholine; SM, sphingomyelin, Sph, sphingosine; Gal-Cer, galactocerebrosides; Cer, ceramide; Chol, cholesterol. A representative of five experiments is shown.
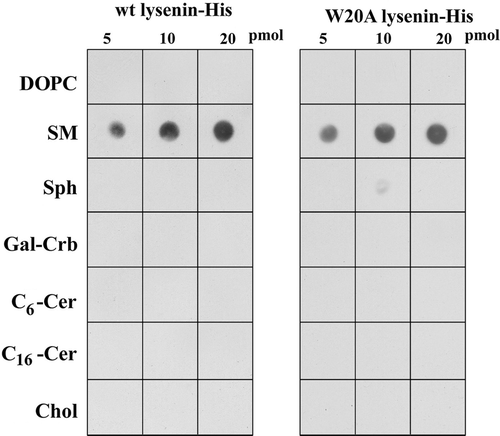
Figure 2. Binding of wt and W20A lysenin to the surface of sheep erythrocytes. Cells were fixed with 1% glutaraldehyde and exposed to the proteins in the presence of 5 mM imidazole, followed by rabbit anti-His IgG and anti-rabbit IgG-FITC. Inset shows cells treated with bacterial sphingomyelinase (70 mU/ml, 1 h, 37°C) before fixation and staining with lysenin. Bar, 10 µm.
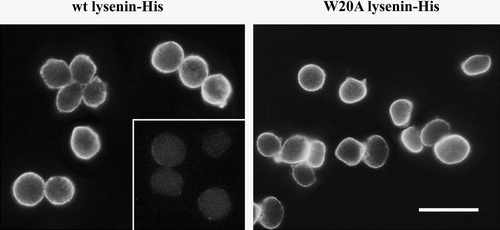
Figure 3. W20A lysenin-His is devoid of hemolytic activity and inefficiently forms oligomers which are characteristic for wt lysenin-His. (A) Hemolytic activity of wt lysenin-His (open squares) and its lack in W20A lysenin-His (closed circles). Sheep erythrocytes (7×107/ml) were incubated with the recombinant proteins at indicated concentrations for 1 h at 4°C. In controls, cells were suspended in H2O to estimate 100% hemolysis. The results are mean±SE from five experiments. (B, C) Wt lysenin-His forms a distinct SDS-resistant oligomer upon binding to sphingomyelin-containing liposomes (B) and sheep red blood cell ghosts (C). (B) Small unilamellar liposomes composed of SM/DOPC with or without cholesterol (1 mM total phospholipids) were incubated with 2.5 µM wt or W20A lysenin-His at the SM/lysenin ratio from 8:1 to 280:1. Pelleted liposomes were subjected to 7% SDS-PAGE under denaturing condition and analyzed for the presence of lysenin monomers and oligomers by immunoblotting with anti-His (upper panel). In lane “no liposomes” 0.2 µg of lysenin-His was applied. On the left, molecular weight standards are shown. Lower panel: Quantification of lysenin monomers (closed symbols) and oligomers (open symbols) based on a densitometric analysis of blots shown in the upper panel. Triangles, liposomes with cholesterol; circles, liposomes without cholesterol. Data are mean±SE from three experiments. (C) Erythrocyte ghosts were incubated with 0.24 µM recombinant proteins in the presence of 0.1–0.8 M NaCl and analysed by SDS-PAGE and immunoblotting with anti-His. Data shown are representative of three experiments.
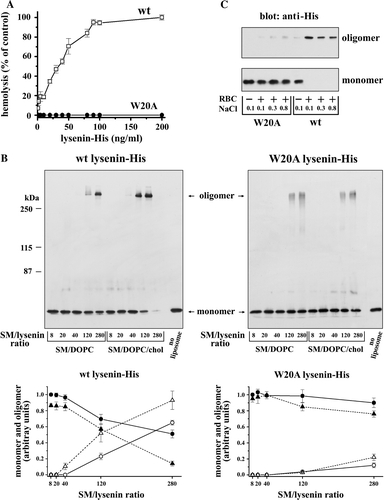
Figure 4. Oligomerization of wt and W20 lysenin-His upon binding to sphingomyelin-containing liposomes analysed by blue native gel electrophoresis and SDS-PAGE (A) and chemical cross-linking with DSS followed by SDS-PAGE (B). Small unilamellar liposomes composed of SM/DOPC/cholesterol (3:7:3, total SM/DOPC 1mM) were incubated with 2.5 µM lysenin (SM/lysenin 120:1). Arrows point to the lysenin monomer and oligomers shown in kDa. Asterisks in (B) indicates aggregates of lysenin remaining on the top of the stacking gel.
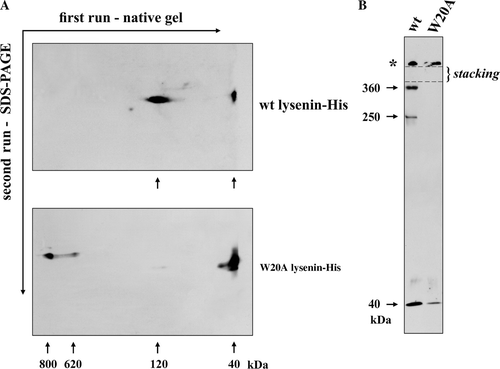
Figure 5. Wild type lysenin-His assembles into a honeycomb-like structure when bound to sphingomyelin-containing liposomes. Recombinant wt lysenin-His (7 µM) was incubated with (A) DOPC/cholesterol (3:2) or (B–D) SM/DOPC/cholesterol mulilamellar liposomes (1:2:2, 6 mM total phospholipids, SM/lysenin 300:1) after which samples were negatively stained and examined under an electron microscope. Bars, 200 nm in (A, B), 50 nm in (C, D). Arrow in (B) indicates lattice formed by wt-lysenin-His.
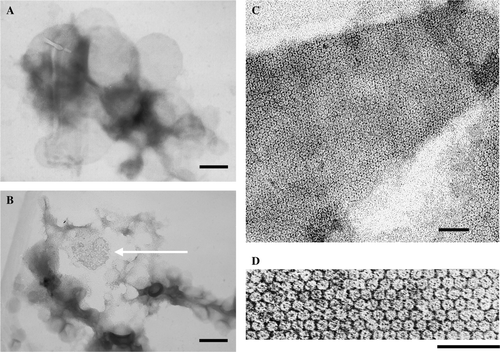
Figure 6. Incorporation of wt lysenin-His into planar lipid bilayers containing sphingomyelin leads to channel formation. All measurements were conducted in asymmetric ionic conditions (450/150 mM KCl, pH 7.0 cis/trans). (A) Wt lysenin-His (1 µg/ml) induces single channel activity recorded in a planar lipid bilayer composed of azolectin/sphingomyelin/cholesterol (96:4:1, by weight) at indicated holding potentials. (B) Time-dependent changes of current induced by 1 µg/ml wt lysenin-His in the azolectin/sphingomyelin/cholesterol bilayer. The measurements were performed continuously for 2 h at 50 mV. (C) Currents recorded in the azolectin/sphingomyelin/cholesterol bilayer in response to a series of wt lysenin-His doses (1–6 µg/ml) within 2 h at the holding potential of 50 mV. (D) Lack of channel activity of wt lysenin-His (2–6 µg/ml) in a planar membrane devoid of sphingomyelin at 50 mV holding potential. (E) W20A lysenin-His (2–6 µg/ml) does not form channels in bilayers composed of azolectin/sphingomyelin/cholesterol at 50 mV. The proteins were added to the trans chamber of experimental cuvettes.
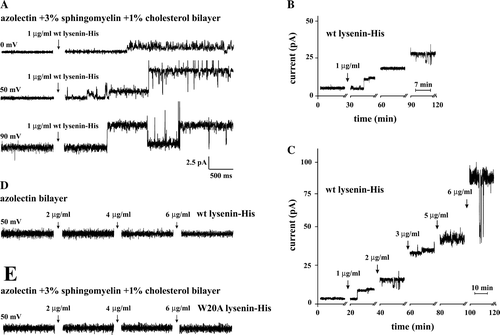
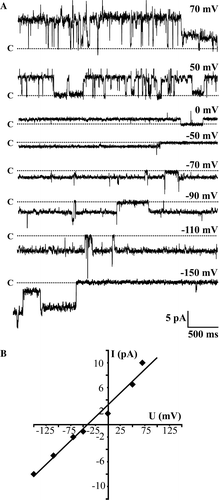
Figure 7. Wild type lysenin-His forms cation-selective channels. (A) Single channel activity induced by wt lysenin-His (1 µg/ml) in an azolectin/sphingomyelin/cholesterol bilayer (96:4:1, by weight) and recorded at indicated holding potentials. Lipid bilayers were exposed to asymmetric ionic conditions (450/150 mM KCl, pH 7.0 cis/trans). All recordings were low–pass filtered at 200 Hz. The closed channel state is indicated by c. (B) Current/voltage relationship of the wt lysenin-His-induced single channel currents from the experiment shown in (A). The current/voltage relationship was linear for holding potentials in the range from −150 mV to 70 mV.
Supplementary Figure 1. Nucleotide sequence of synthetic lysenin gene. The coding strand synthetic oligonucleotides are in capital and lower-case letters, alternately, and are marked by symbols “Ux” on the right margin of the same row; oligonucleotides joining the upper ones during ligation are written in bold capital letters and marked by symbols “Jx_x + 1” on the right margin. The amino acid sequence of lysenin is given above the coding upper strand. Start and stop codons are in bold italics. The nucleotide sequence of oligonucleotide P2 which allowed amplification of the final PCR product is double underlined. Tryptophan 20 that was substituted with alanine in W20A lysenin-His is indicated by black background.
