Figures & data
Figure 1. Jahnig profile of the Cter domain (400–522) of colicin E1. The increasing oscillating curve corresponding to the H8 helix (residues 471–487) and the decreasing oscillating curve corresponding to H9 (residues 494–510) are indicated.
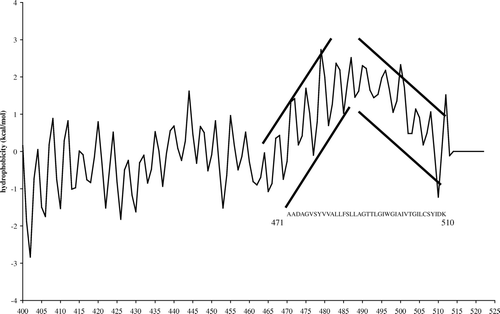
Figure 2. Most stable position of H8 (A) and H9 (B) in the IMPALA membrane after the angular dynamics. Mid plane = bilayer centre (z = 0); first upper (beneath) plane = lipid acyl chain/polar headgroup interface at 13.5 Å from the centre; second upper (beneath) plane = lipid/water interface (z = 18 Å).
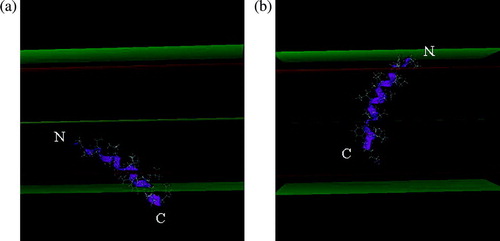
Table I. Conformation of the H8 and H9 peptides in 100% TFE and in the presence of lipids.
Figure 3. Time course of lipid mixing induced by H8 and H9 peptides. Liposomes (SUV) are a mixture of 26.6% PC, 26.6% PE, 26.6% SM and 20.2% cholesterol (black symbols-called NC SUV below) or of 49.5% DOPC, 50% DPPC and 0.5% DOPA (open symbols-called DOPC SUV below). Peptides are added at 150 µM (corresponding to a peptide to lipid molar ratio of 1/10) to a mixture (1:4 w/w ratio) of R18-labelled and unlabelled SUVs. Increase of the R18 relative fluorescence is followed at room temperature. The SIV tilted peptide at 150 µM is used as positive control; the contribution of TFE (1.6% final concentration) is substracted from the curves. 100% fusion is obtained by adding 2% Triton X-100 to liposomes. ♦ H9/NC SUVs; ◊ H9/DOPC SUV; + H8/NC SUV; * H8/DOPC SUV; • SIV/NC SUV; ○ SIV/DOPC SUV.
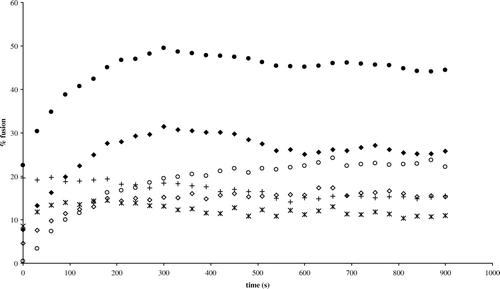
Table II. Percentage of lipid mixing induced by H9 and H8 in the presence of LUV or SUV (PC/PE/SM/chol as described in methods). 100% corresponds to the fusion induced by the SIV tilted peptide in the same conditions.
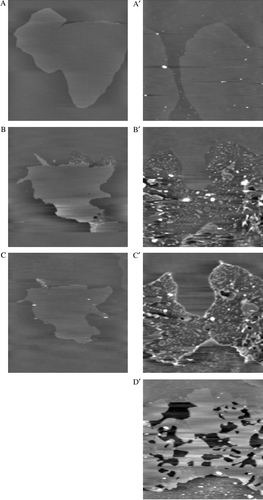
Figure 4. Influence of colicin H8 (A, B and C) and H9 (A' to D') peptides on DOPC/DPPC/DOPA bilayers. AFM topographic images (A–C, 15 µm×15 µm; A'–D', 20 µm×20 µm; z- range: 10 nm) of a DOPC/DPPC/DOPA (495/500/5; mol/mol/mol) bilayer recorded in Tris/NaCl/EDTA, pH7.4 (A and A'). Images of the same bilayer was acquired in the presence of a 10 µM peptide solution (Tris/NaCl/EDTA): H8, after 3 (B) and 10 min (C); H9, after 5 (B'), 15 (C') and 120 min (D').