Figures & data
Table 1. Antibiotic susceptibility patterns of coagulase-positive staphylococci and coagulase-negative staphylococci isolated from bovine mastitis.
Figure 1. Time kill assay of YSMP (a), A. catechu Linn. (b), C. longa Linn. (c), G. mangostana Linn. (d), curcumin (e), and α-Mangostin (f) against S. epidermidis ATCC 35984, coagulase-negative staphylococci NPRC BCNS018, and coagulase-positive staphylococci NPRC BCPS031. MHB containing 1% of DMSO was added as a positive control. MICs of YSMP, A. catechu, C. longa, G. mangostana, α-Mangostin, and curcumin against S. epidermidis ATCC 35984/BCNS018/BCPS031 were 7/15/31, 250/250/250, 125/250/250, 3/7/7, 1/10/10, and 31/250/250 µg/mL, respectively.
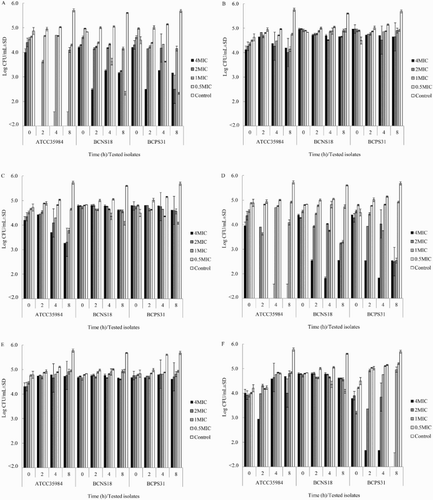
Table 2. MICs of ethanol extracts of YSMP, its herbal components, and active constituents against bovine mastitis-isolated coagulase-positive staphylococci (BCPS) and coagulase-negative staphylococci (BCNS).
Table 3. Biofilm-forming ability of coagulase-positive staphylococci and coagulase-negative staphylococci isolates.
Figure 2. Effect of different concentrations of YSMP (a), G. mangostana (b), and α-Mangostin (c) on the bacterial growth (white bars) and biofilm formation (black bars) of coagulase-positive staphylococci NPRC BCPS031. Note: Each bar indicates the percentage of the means of inhibition ± SE for three independent experiments performed in duplicate.
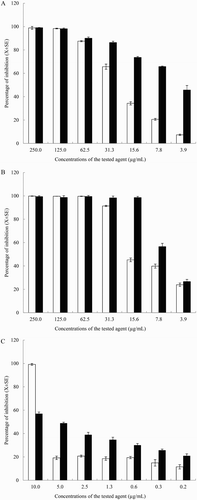
Figure 3. Scanning electron micrograph of BCNS 18 after treated with four MIC of YSMP (b), G. mangostana (c), A. catechu (d), C. longa (e), and alpha-mangostin (f). BCNS 18 (a) was growth in TSB used as a control. MICs of YSMP, G. mangostana, C. longa, A. catechu, and alpha-mangostin against BCNS 18 were 15, 7, 250, 250, and 10 µg/mL, respectively.
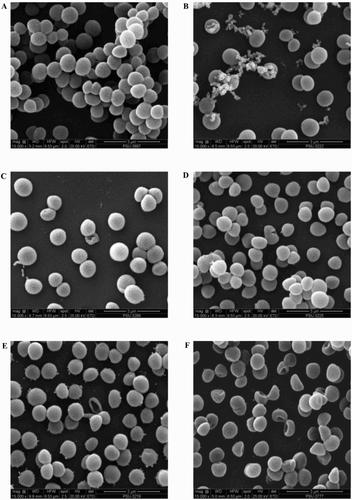
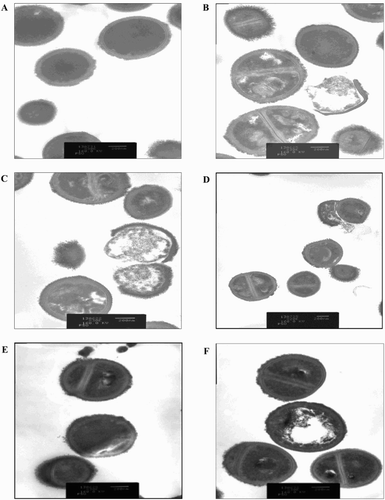
Figure 4. Transmission electron micrograph of BCNS 18 after treated with four MIC of YSMP (b), G. mangostana (c), A. catechu (d), C. longa (e), and alpha-mangostin (f). BCNS 18 (a) was growth in TSB used as a control. MICs of YSMP, G. mangostana, C. longa, A. catechu, and alpha-mangostin against BCNS 18 were 15, 7, 250, 250, and 10 µg/mL, respectively.