Figures & data
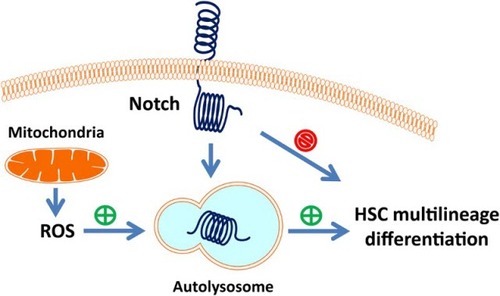
Figure 1 Mitochondrial ROS promotes hematopoietic stem cell differentiation but attenuates Notch signaling. (A) ROS generation is progressively increased during hematopoietic stem cell differentiation (LSK, LK, Lin- and Lin+) measured by flow cytometry. **P < 0.01; ns, P > 0.05. (B) Overlays of mitochondrial membrane potential stained with JC-1 in WT mice LSK, LK, Lin- and Lin+ cells. (C) Indicated cells were stained with 5 μM MitoSOX Red, specific for mitochondrial O2−·, for 15 minutes and then analyzed flow cytometry. Percentage of MitoSox Red positive cells was quantified. **P < 0.01. (D, E) NAC and MitoQ inhibited rapamycin-induced differentiation measured by benzidine staining. Percentage of benzidine-positive cells was quantified (right panel). **P < 0.01; ns, P > 0.05. (F) Comparison of in vitro CFU-C in control and MitoQ treated hematopoietic stem and progenitor cells (LSK) and counted on day 7. The numbers of colonies were quantified (right panel). *P < 0.05. (G) Blood routine examination of peripheral blood form control and NAC treated wild-type mice. *P < 0.05. (H) Cytometric measurement of intracellular Notch protein level in LSK cells isolated from wild-type mice treated with or without NAC. *P < 0.05. (I) qRT-PCR detection of the expression for Notch target genes Hey2 and Cyclin D1 in LSK cells isolated from NAC treated wild-type mice. *P < 0.05; **P < 0.01.
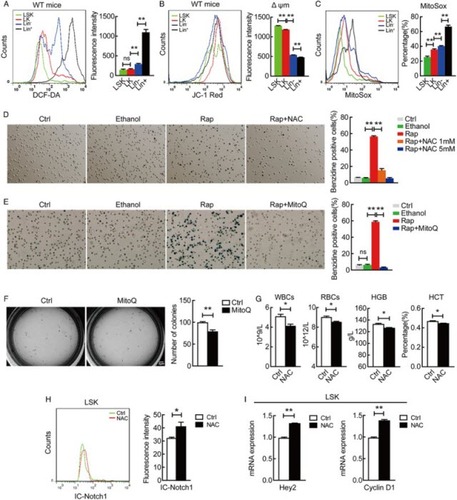
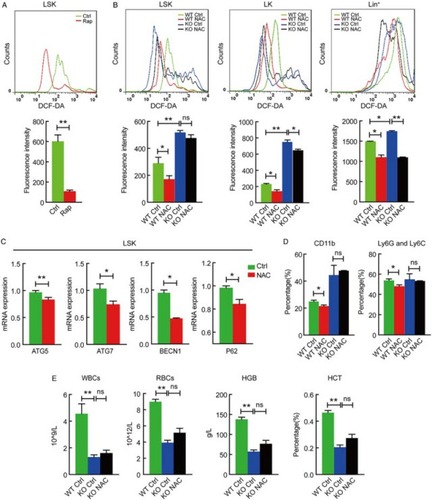
Figure 2 ROS regulates hematopoietic stem cell differentiation in an autophagy-dependent manner. (A) Rapamycin reduced ROS level in wild-type mouse LSK cells, measured by DCF-DA staining. Results were quantified (lower panel). **P < 0.01. (B) Autophagy defect in different stages (LSK, LK and Lin+) of hematopoietic hierarchy caused elevated ROS generation and different response to NAC treatment. *P < 0.05; **, P < 0.01; ns, P > 0.05. (C) qRT-PCR detection of the expression for autophagy-essential genes in LSK cells isolated from NAC treated or not treated wild-type mice. *P < 0.05; **P < 0.01. (D) NAC reduced the percentage of CD11b and Ly6G-Ly6C bone marrow cells in wild-type, not Atg7−/− mice, measured by flow cytometry. **P < 0.01, *P < 0.05. (E) Autophagy defect impaired multilineage differentiation and caused a failure in response to NAC treatment. Wild-type and Atg7−/−mice were treated with or without NAC, and peripheral blood was counted **P < 0.01; ns, P > 0.05. All experiments were repeated at least three times. n ≥ 5.