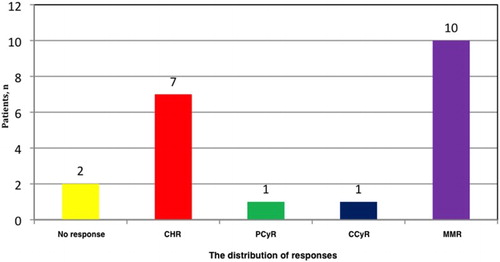
Figures & data
Figure 1. The consort diagram showing the study cohort and treatment outcomes (2G, second generation; BC, blast crisis; CCyR, complete cytogenetic response; CML-CP, chronic myeloid leukemia in chronic phase; DAS, dasatinib; IM, imatinib; MMR, major molecular response; NIL, nilotinib; TKI, tyrosine kinase inhibitor). *One patient in each group died due to non-CML related causes while they were in MMR, the others deceased due to disease progression.
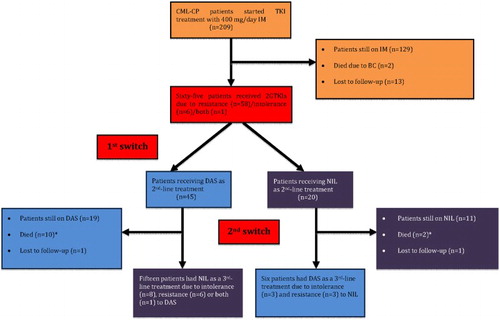
Table 1. Baseline characteristics and treatment outcomes of patients who received third-line 2GTKIs.
Figure 3. The event-free (A) and overall (B) survivals of patients when cases were divided into two according to the reason of switching to third-line TKI therapy as patients with failure (PF; n = 9) and intolerance (PI; n = 11) [OS was calculated from the date of diagnosis until the time of death or last follow-up. EFS was calculated from the onset of third-line TKI therapy until the date of any event defined].
![Figure 3. The event-free (A) and overall (B) survivals of patients when cases were divided into two according to the reason of switching to third-line TKI therapy as patients with failure (PF; n = 9) and intolerance (PI; n = 11) [OS was calculated from the date of diagnosis until the time of death or last follow-up. EFS was calculated from the onset of third-line TKI therapy until the date of any event defined].](/cms/asset/48cab256-6b3c-43e0-8e83-cd3c0953d7da/yhem_a_1385193_f0003_c.jpg)