Figures & data
Figure 1. Experiment 1: Effect of stress and sex on plasma corticosterone concentrations. Plasma corticosterone concentration was increased by 30 min of acute restraint stress. Females had greater plasma corticosterone concentrations compared to males during both basal and stressed conditions. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). See for statistical details.
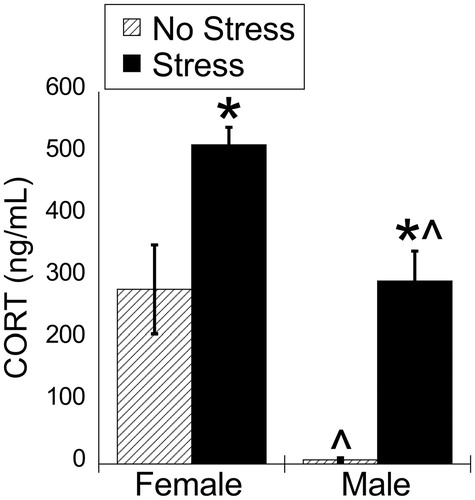
Table 1. Two-way ANOVA results for Experiment 1.
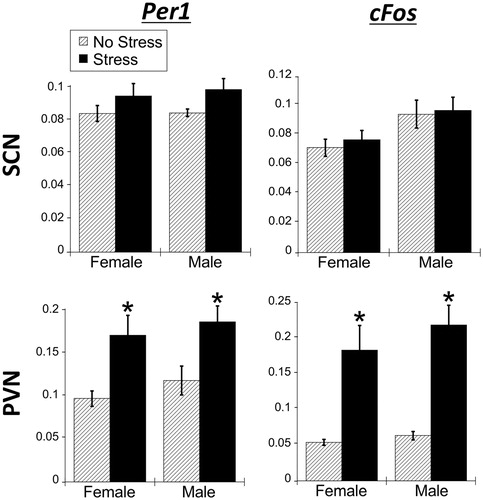
Figure 2. Experiment 1: Effect of stress and sex on gene expression in the SCN. (A) There were no significant main effects of stress or sex for Per1, Per2, and Bmal1 mRNA in the suprachiasmatic nucleus (SCN) of male and female rats. cFos mRNA was increased by stress in only male SCN. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). (B and C) Representative autoradiographs of gene expression under no stress and stress conditions of female (B) and male (C) rats. The SCN is located within the box. See for statistical details.
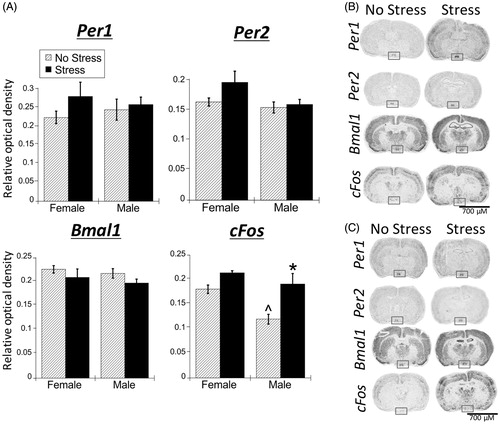
Figure 3. Experiment 1: Effect of stress and sex on gene expression in the PVN. (A) 30 min of acute restraint stress increased Per1 and cFos mRNA in the paraventricular nucleus of the hypothalamus (PVN) of male and female rats. There were no sex differences in stress-induced Per1 and cFos mRNA. Stress also increased Per2 mRNA only in female rat PVN. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). (B and C) Representative autoradiographs of gene expression under no stress and stress conditions of female (B) and male (C) rats. The PVN is located within the box. See for statistical details.
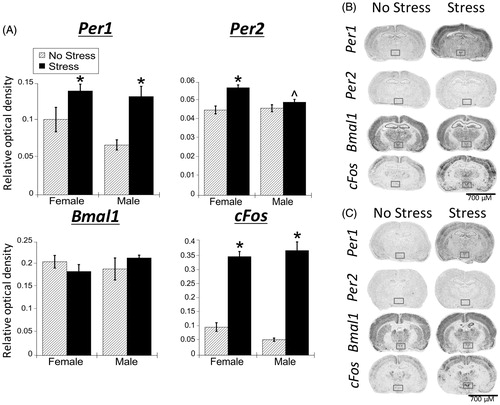
Figure 4. Experiment 1: Effect of stress and sex on gene expression in the PFC and rostral agranular insula (RAI). (A) Acute stress increased Per1 and cFos mRNA throughout the prefrontal cortex (PFC) subregions (anterior cingulate, AC; prelimbic cortex, PL; infralimbic cortex, IL; ventral orbital cortex, VO), and to a lesser extent in the RAI, of male and female rats. Per2 mRNA was also increased by stress, but only in the VO subregion. There was no effect of stress on Bmal1 mRNA. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress condition; p < .05, FLSD, n = 6 rats per treatment group). (B and C) Representative autoradiographs of gene expression under no stress and stress conditions of female (B) and male (C) rats. See for statistical; details.
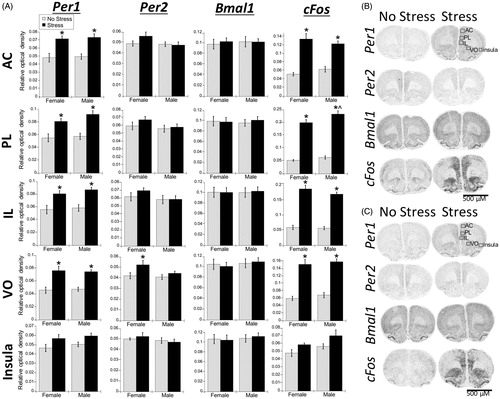
Table 2. Two-way ANOVA results for Experiment 2.
Figure 5. Experiment 2: Effect of stress and adrenal status on gene expression in the SCN. (A) Acute restraint stress had no effect on Per1, Per2, and Bmal1 mRNA expression. Stress increased cFos mRNA in the suprachiasmatic nucleus (SCN) of SHAM rats only. Data are presented as mean ± SEM (*stress effect within same adrenal status conditions [sham or adrenalectomized]; p < .05, FLSD, n = 4–7 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of SHAM (B) and adrenalectomized (ADX) (C) rats; the SCN is located within the box. See for statistical details.
![Figure 5. Experiment 2: Effect of stress and adrenal status on gene expression in the SCN. (A) Acute restraint stress had no effect on Per1, Per2, and Bmal1 mRNA expression. Stress increased cFos mRNA in the suprachiasmatic nucleus (SCN) of SHAM rats only. Data are presented as mean ± SEM (*stress effect within same adrenal status conditions [sham or adrenalectomized]; p < .05, FLSD, n = 4–7 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of SHAM (B) and adrenalectomized (ADX) (C) rats; the SCN is located within the box. See Table 2 for statistical details.](/cms/asset/bd0999d9-01dc-481a-a3d2-a492edb7f979/ists_a_1404571_f0005_b.jpg)
Figure 6. Experiment 2: Effect of stress and adrenal status on gene expression in the PVN. (A) Acute restraint stress increased Per1 and cFos, but not Per2 or Bmal1 mRNA in the paraventricular nucleus of the hypothalamus (PVN), replicating the male data from Experiment 1. Stress increased cFos mRNA occurred regardless of adrenal status (sham or adrenalectomized). However, stress-induced Per1 mRNA was attenuated by adrenalectomy (ADX). Data are presented as mean ± SEM (*stress effect within same adrenal status condition; &adrenal status effect within same stress conditions; p < .05, FLSD, n = 5–6 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of sham (B) and ADX (C) rats; the PVN is located within the box. See for statistical details.
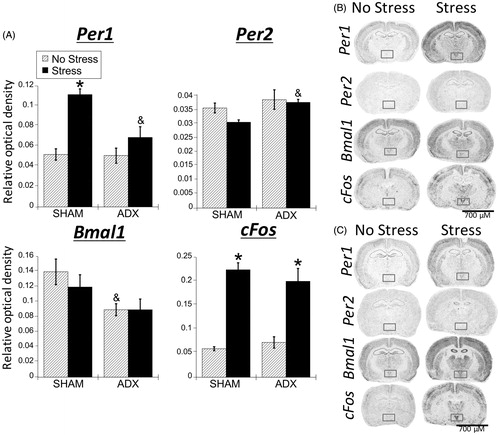
Figure 7. Experiment 2: Effect of stress and adrenal status on gene expression in the PFC and rostral agranular insula (RAI). (A) Acute stress increased Per1 and cFos mRNA throughout the prefrontal cortex (PFC) subregions (anterior cingulate, AC; prelimbic cortex, PL; infralimbic cortex, IL; ventral orbital cortex, VO), but not in the RAI. In the AC and PL subregions, stress increased Per1 mRNA in both SHAM and adrenalectomized (ADX) rats, suggesting corticosterone-independent effects. The VO subregion had stress-induced Per1 mRNA only in SHAM rats. cFos mRNA was induced by stress regardless of adrenal status, but the increase was attenuated by adrenalectomy in the PL and IL. There was also a main effect for stress to decrease Bmal1 mRNA levels in the IL, VO, and RAI. Data are presented as mean ± SEM (*stress effect within same adrenal status conditions; &adrenal status effect within same stress conditions; p < .05, FLSD, n = 5–6 rats per treatment group). (B and C) Representative autoradiographs under no stress or stress conditions of sham (B) and ADX (C) rats. See for statistical details.
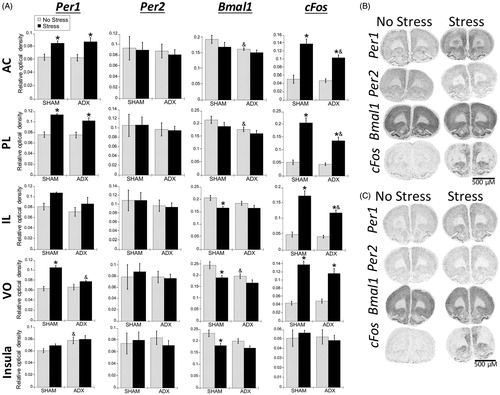
Figure 8. Experiment 3: Effect of stress at zeitgeber time (ZT)16 on plasma corticosterone concentrations in male and female rats. 30 min of acute restraint stress increased plasma corticosterone concentrations in both male and female rats. Data are presented as mean ± SEM (*stress effect within same sex condition; ^sex effect within same stress conditions; p < .05, FLSD, n = 6 rats per treatment group). See for statistical details.
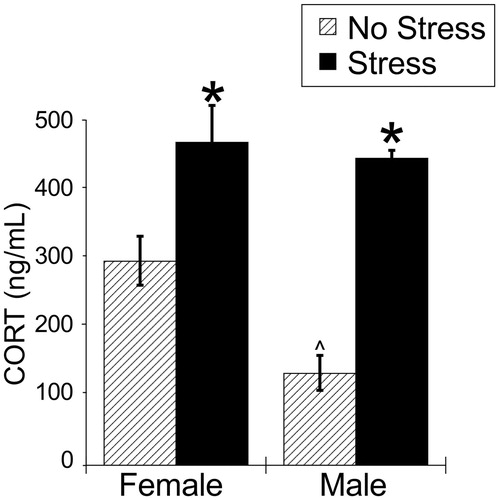
Table 3. Two-way ANOVA results for Experiment 3.
Figure 9. Experiment 3: Effect of stress at ZT16 on SCN and PVN Per1 and cFos mRNA in male and female rats. When rats were exposed to 30 min of acute restraint stress at ZT16, there was no increase of Per1 and cFos mRNA in the suprachiasmatic nucleus (SCN) of both male and female rats. However, in the paraventricular nucleus of the hypothalamus (PVN), both males and females showed a significant increase in both Per1 and cFos mRNA. Data are presented as mean ± SEM (*stress effect within same sex conditions, p < .05, FLSD, n = 6 rats per treatment group). See for statistical details.