Figures & data
Figure 1. Experimental design. Animals were weaned at post-natal day (p)21 and assigned to control or peripuberty stress groups. The stress protocol consisted of exposure to an open field (OF) and elevated platform (EP) on p28, followed by intermittent, variable exposure to an EP and predator odor (trimethylthiazoline, TMT) until p42. Control animals were handled briefly on the days on which their experimental counterparts were exposed to stress. Behavioral testing started at p90, with a minimum delay of one week imposed between tests. 2-DG: 2-deoxyglucose.
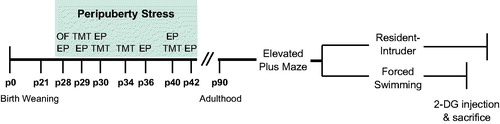
Figure 2. Corticosterone response to repeated stressor exposure across days during the peripubertal period differed in accordance with selection line. Low- and high-line rats did not differ in their response to a first exposure to stress on p28. Thereafter, low-line rats showed habituation of the corticosterone response to stressors on p30 and p42, an effect attenuated in high-line rats (A). These differences were reflected by corticosterone concentration in a second plasma sample, taken 30 minutes after the offset of stress (B). In addition to strong habituation across stressors, low-line rats showed accelerated recovery of the HPA axis following the first stress session on postnatal (p) day 28 (two-way RM ANOVA: interaction is represented by an uncapped line; independent sample t-test results are above each timepoint; *p < .05, ***p < .001; see text for details).
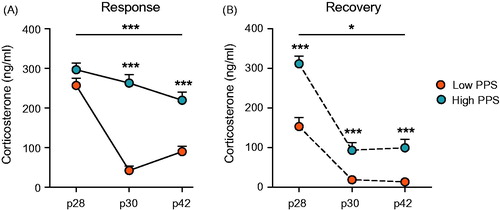
Figure 3. Anxiety-like behavior on the elevated plus maze. Low-line rats spent less time in the closed arm of the maze, and more time on the open arm when compared to high-line rats (A). Following exposure to peripuberty stress (PPS), rats spent more time in the closed arm and less time in the open arm of the maze. Additionally, stressed rats showed a decrement in locomotion in terms of distance covered on the maze (B) in comparison to non-stressed controls (two-way ANOVA: main effect of treatment is represented by a capped line; main effect of line is represented by a line with feet; *p < .05; **p < .01; ***p < .001; see text for details).
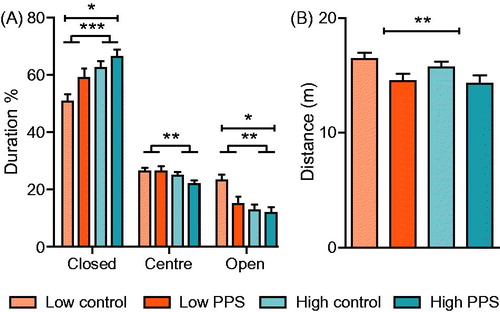
Figure 4. Depression-like behavior in the forced-swimming test. Neither selection line, nor prior exposure to peripuberty stress (PPS), influenced behavioral coping response to the first exposure to forced-swimming (A). When re-exposed to this stressor on the following day, compared with low-line rats, high-line rats spent significantly more time engaged in passive coping, as indexed by time spent floating (B) (two-way ANOVA: main effect of line; *p < .05; see text for further details).
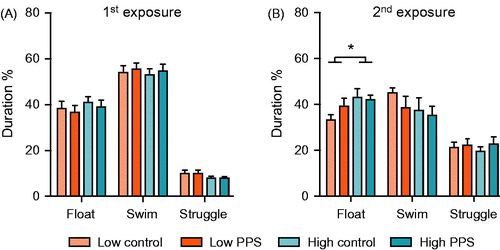
Figure 5. Aggression in the resident–intruder test. Compared to low-line rats, high-line rats spent a greater duration of the test engaged in offensive behaviors (A) and attacked more frequently (C). There was an interaction between selection line and experience of peripuberty stress (PPS) in terms of readiness to engage in offensive behavior (C), with low-line PPS rats tending to be quicker to attack than low-line controls. When these three measures were combined (D), an interaction between selection line and treatment revealed that PPS exposure increased the overall aggressiveness of low-line rats, while not altering relative aggressiveness of high-line rats. Low-line rats were more likely to perform abnormal forms of aggression (E; diagonally striped bars), the proportion of rats performing “normal” forms of attack was similar between groups (solid bars) (two-way ANOVA: main effect of line is represented by a line with feet; post hoc t-tests by a narrow, uncapped line; *p < .05; **p < .01; see text for further details).
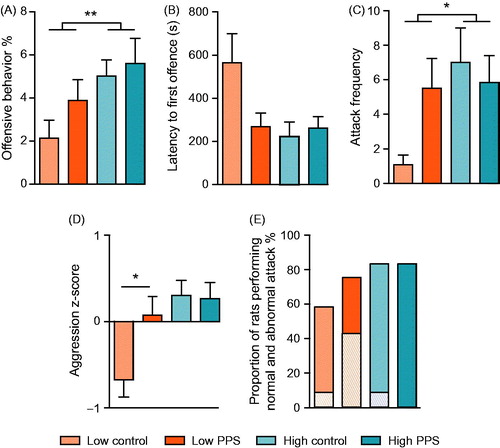
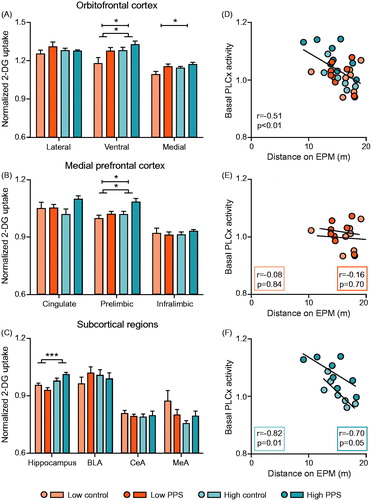
Figure 6. Quantification of brain activity under basal conditions, as indexed by uptake of 2-deoxyglucose (2-DG), in stress-sensitive limbic brain regions. In ventral and medial subdivisions of the orbitofrontal cortex (A), relative to low-line rats, high-line rats had enhanced 2-DG uptake. Moreover, PPS exposed rats showed enhanced 2-DG uptake relative to controls in those same regions. This pattern was mirrored in the prelimbic division of the medial prefrontal cortex (B) but not in other subregions. In hippocampus (C), high-line rats again had higher 2-DG uptake than low-line rats. Levels of prelimbic cortex activity under basal conditions were associated with locomotion on the EPM (D–F) (two-way ANOVA: main effect of treatment is represented by a capped line, main effect of line by a line with feet; *p < .05; ***p < .001; see text for details). FST: forced-swimming test; EPM: elevated plus maze; vOFC: ventral orbitofrontal cortex; mOFC: medial orbitofrontal cortex; PLCx: prelimbic cortex; BLA: basolateral amygdala; CeA: central nucleus of the amygdala; MeA: medial nucleus of the amygdala.