Figures & data
Figure 1. Experimental design summaries for Experiments 1-3. CORT: plasma corticosterone measured in blood sample taken via tail nick except for D9 blood sample taken via trunk blood; D: Testing day; Env, (Novel) Environment testing.
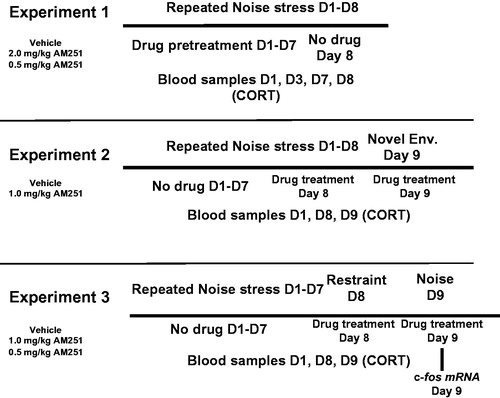
Figure 2. Dose-dependent ability of CB1 receptor antagonism disrupts the expression, but not the acquisition, of CORT response habituation to repeated loud noise stress. Rats (n = 8/group) were given systemic pretreatment with CB1 receptor antagonist AM251 (2.0 or 0.5 mg/kg) or vehicle before each of the first 7 days of loud noise stress exposure (30 min/day, 95 dB). Repeated Measures (RM) two-way ANOVA indicated that 2.0 mg/kg AM251 treatment significantly potentiated plasma corticosterone (CORT) responses and resulted in slower rate of habituation (# significant interaction of stress and drug, p < 0.001, confirmed post hoc with Bonferroni mct). Rats were not given drug treatment before the 8th loud noise stress exposure in which all three groups displayed similar level of HPA axis response habituation. The 2.0 mg/kg AM251 treatment group displayed significant reduction from day 7 to day 8, but 0.5 mg/kg AM251 and vehicle treatment groups did not (*p < 0.001).
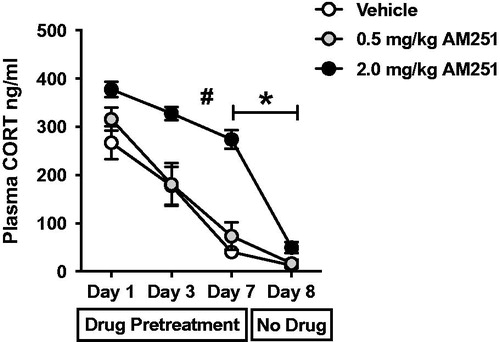
Figure 3. Low dose CB1 receptor antagonist AM251 (1 mg/kg) pretreatment disrupts the expression of HPA axis habituation. Test of CB1 receptor involvement in heterotypic stressor sensitization. (A) Rats (n = 9/group) were exposed to daily loud noise stress without drug treatment (30 min/day, 95 dB). A group of control rats (n = 3) received similar handling but were not exposed to noise stress during the first 8 days, so serve as a control for day 9 testing (n = 3) included for day 9 control group. Repeated measures two-way ANOVA indicates that pretreatment with 1.0 mg/kg AM251 before the 8th loud noise exposure completely prevented expression of a habituated HPA axis response. Vehicle-treated controls display robust habituation of plasma CORT response to the 8th loud noise exposure, compared to the 1st exposure (^p < 0.001, post hoc Bonferroni mct). 1 mg/kg AM251 treatment results in significantly higher plasma CORT response to the 8th loud noise exposure, compared to vehicle controls (*p < 0.001, post hoc Bonferroni mct). (B) Heterotypic stressor sensitization test: After 8 days of loud noise stress, rats were exposed to 15 minutes of novel environment stress on day 9. 1 mg/kg AM251 treatment significantly increased plasma CORT levels compared to vehicle-treated rats with the same stress history (*p < 0.05, post hoc Bonferroni mct). A lack of facilitating effect on plasma CORT response to 1.0 mg/kg AM251 was observed in control rats without recent stress history when compared to their previous non-stress values or to vehicle treated rats with repeated stress history. However, the difference between the two 1.0 mg/kg-treated groups failed to reach significance (p > 0.05, post hoc Bonferroni mct).
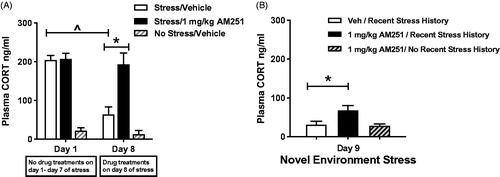
Figure 4. Low doses of CB1 receptor antagonism partially disrupt the expression of CORT response habituation to loud noise stress, and result in sensitized response to a heterotypic stressor. (A) Vehicle and 0.5 mg/kg AM251 treatment groups display significant habituation on Day 9 (^p < 0.05, RM two-way ANOVA with Bonferroni post hoc mct). 1.0 mg/kg AM251 significantly reduced the expression of habituation on Day 9 (*p < 0.05, planned t-test comparison). 0.5 mg/kg treatment did not reach significance in this measure (p = 0.1, planned t-test comparison). (B) Plasma CORT response to repeated noise stress graphed as percentage of Day 1 response. Both doses of AM251 significantly reduced the percentage of habituation, indicating a disruption of the expression of habituation (*p < 0.05, planned t-test comparisons). (C) Day 8 cross-sensitization testing. Repeatedly stressed rats displayed significantly increased plasma CORT response to restraint, compared to vehicle-treated group (*p < 0.05). (D) Acute noise and restraint stress controls. 0.5 mg/kg AM 251 did not potentiate plasma CORT response to the initial loud noise exposure in acute stress control rats (p = 0.34, t-test). Tissue was collected from rats after acute noise stress for comparisons of neural activity to repeatedly stressed rats. In a separate cohort of rats without recent repeated stress history, 0.5 mg/kg AM251 did not potentiate plasma CORT response to restraint stress (p = 0.75, t-test).
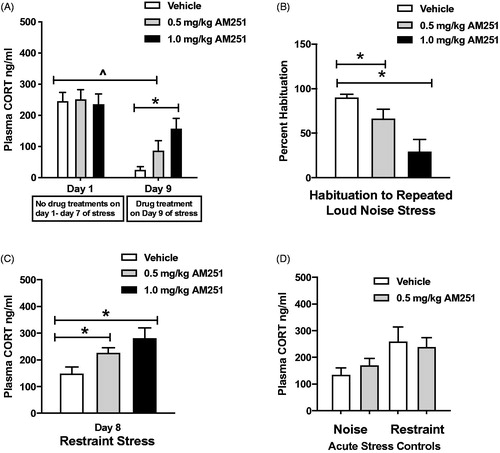
Figure 5. Representative autoradiographs of c-fos mRNA with corresponding atlas images (Paxinos and Watson). These representative autoradiographs indicate the regions quantified, as well as the template size, shape, and placement used in analysis. (A) Orbitofrontal cortex (OF). (B) top: Prelimbic cortex (PL), bottom: Infralimbic cortex (IL). (C) Lateral Septum (LS). (D) Antero-ventral bed nucleus of the stria terminalis (BSTav). (E) Paraventricular nucleus of the hypothalamus (PVN). (F) top/medial: Central nucleus of the amygdala (CeA), bottom/lateral: Basolateral nucleus of the amygdala (BLA). (G) Rostral posterior hypothalamus (rPH). (H) Auditory cortex.
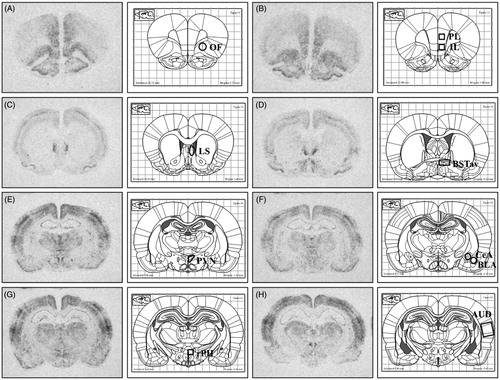
Figure 6. Low dose CB1 receptor antagonism disrupts the habituation of neural activity in multiple limbic regions. Stress-induced c-fos mRNA significantly habituated in the A. Paraventricular nucleus of the hypothalamus (PVN), B. Rostral posterior hypothalamus (rPH), C. Lateral septum (LS), and in the D. Anteroventral bed nucleus of the stria terminalis (BSTav) after 8 exposures to repeated loud noise stress (^p < 0.05, two-way ANOVA). 0.5 mg/kg AM251 did not significantly increase c-fos mRNA responses to initial, acute loud noise stress, but this dose significantly disrupted the expression of c-fos mRNA habituation in the (A) PVN, (B) rPH, and (C) LS (*p < 0.05 planned t-test comparisons), but this measure did not reach significance in the (D) BSTav (p = 0.1). A significant interaction of drug and stress experience was measured in the PH (#p < 0.05, two-way ANOVA). BLA and CeA c-fos mRNA did not display significant increase by CB1 receptor antagonism (BLA: p = 0.1, CeA: p = 018, planned t-test comparisons).
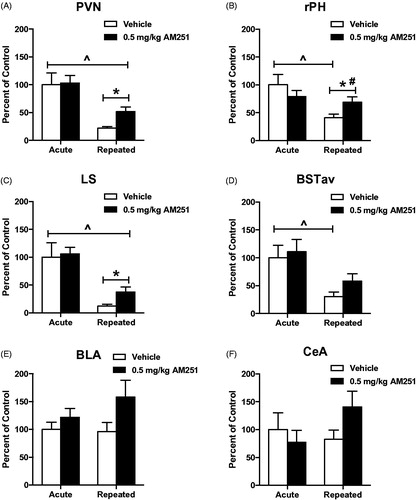
Figure 7. Representative autoradiographs of c-fos mRNA for neural regions with disruption of habituated response by low dose CB1 receptor antagonism. These images demonstrate the autoradiographic pattern observed in the neural regions in which a low dose of CB1 receptor antagonist AM251 (0.5 mg/kg, intraperitoneal injection) was not measured to potentiate expression of the immediate early gene resulting from a single acute loud noise stress exposure, but did significantly disrupt the expression of habituation of this marker of neural activity compared to vehicle-treated controls. LS: Lateral septum, PVN: Paraventricular nucleus of the hypothalamus, rPH: Rostral posterior hypothalamus (regions generally indicated with arrows).
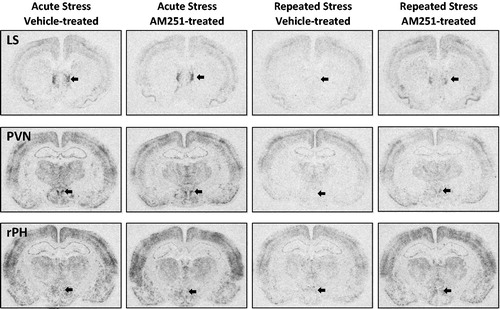
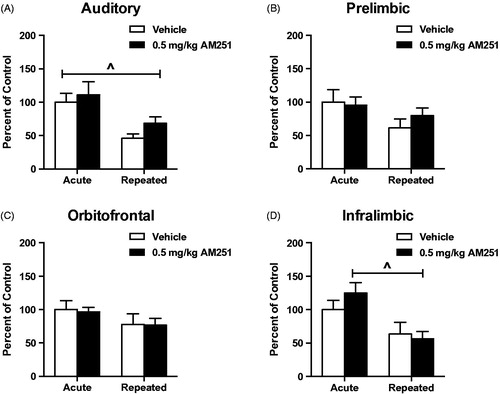
Figure 8. Low dose CB1 receptor antagonism does not alter responses of cortical neural activity in rats habituated to loud noise stress. (A) Auditory cortex and (D). Infralimbic cortex c-fos mRNA were measured to significantly habituate after 8 loud noise stress exposures (^p < 0.001, two-way ANOVA). (B) Prelimbic and (C). Orbitofrontal (C) cortex c-fos mRNA did not significantly habituate to repeated loud noise stress (p > 0.05, two-way ANOVA). Low dose CB1 receptor antagonism did not alter c-fos mRNA responses in rats habituated to loud noise stress in any of these cortical regions (p > 0.05, planned t-test comparisons).