Figures & data
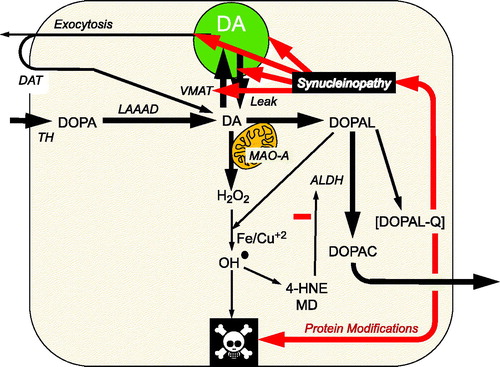
Figure 1. Overview of the catecholaldehyde hypothesis. Dopamine (DA) is synthesized from 3,4-dihydroxyphenylalanine (DOPA) by l-aromatic-amino-acid decarboxylase (LAAAD), after DOPA is produced from tyrosine via tyrosine hydroxylase (TH). DOPAL is formed from the action of monoamine oxidase-A (MAO-A) on cytoplasmic dopamine (DA). DOPAL is detoxified by aldehyde dehydrogenase (ALDH), which converts DOPAL to 3,4-dihydroxyphenylacetic acid (DOPAC). DOPAC rapidly exits the cell. According to the catecholaldehyde hypothesis, interactions of DOPAL and the protein alpha-synuclein set the stage for vicious cycles that challenge homeostasis in catecholaminergic neurons. DOPAL oxidizes spontaneously to DOPAL-quinone (DOPAL-Q). DOPAL reacts with hydrogen peroxide and divalent metal cations to form hydroxyl radicals, which peroxidate membrane lipids. The lipid peroxidation products 4-hydroxynonenal (4-HNE) and malondialdehyde (MD) inhibit ALDH. DOPAL, probably via oxidation to DOPAL-Q, oligomerizes and forms quinoprotein adducts with (“quinonizes”) AS. DOPAL-induced synuclein oligomers impede vesicular functions, evoking destabilizing positive feedback loop.