Figures & data
Figure 1. Overlapping neural circuits mediating HPA-axis “stress responsivity” and individual differences in cue-motivated behaviors. The key elements mediating the hypothalamic-pituitary-adrenal (HPA)-axis “stress response” are depicted (in black), and start with the conversion of neural information from the prefrontal cortex (PFC), hippocampus (Hipp), amygdala (Amy), and brainstem at the paraventricular nucleus (PVN) of the hypothalamus. Information is relayed directly to the PVN or indirectly via the bed nucleus of the stria terminalis (BNST). The HPA axis is triggered with the release of corticotropin releasing hormone (CRH) from the PVN. Consequently, ACTH secretion from the pituitary is elicited; and, subsequently, the synthesis and secretion of glucocorticoids from the adrenal cortex occurs. Glucocorticoids are diffused across the body and brain. At baseline levels, they exert their effects upon Type I-MRs (in green) located primarily within the hippocampus (Hipp) and less so within the PFC, Amy, and paraventricular nucleus of the thalamus (PVT). Under stress or circadian peak, they activate Type II-GRs (in yellow) located ubiquitously across the pituitary, PVN, PVT, Amy, PFC, Hipp, lateral septum (LS), nucleus accumbens (NAc), ventral tegmental area (VTA), nucleus tractus solitarus (NTS), locus coeruleus (LC), and cerebellum (Cb). (Ahima & Harlan, Citation1990; Chao et al., Citation1989; Fuxe et al., Citation1985; Citation1985; Jaferi & Bhatnagar, Citation2006; Reul & de Kloet, Citation1985). Structures that comprise the “motive circuit” (Kalivas & Volkow, Citation2005; Kelley et al., Citation2005) and have been implicated in individual differences in cue-learning (Flagel & Robinson, Citation2017) are shown in light beige. This includes: medial PFC(mPFC) (Campus et al., Citation2019; Haight et al., Citation2017), orbitofrontal cortex (OFC) (Stringfield et al., Citation2017), Hipp (Fitzpatrick et al., Citation2016), NAc (Saunders & Robinson, Citation2012), ventral pallidum (VP) (Ahrens et al., Citation2016), Amy (Flagel et al., Citation2011), lateral hypothalamus (LH) (Haight et al., Citation2017), PVT (Campus et al., Citation2019), and VTA (Flagel et al., Citation2011).
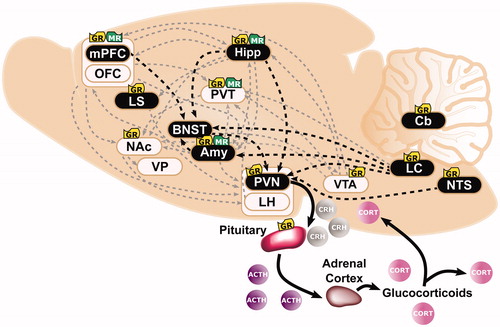
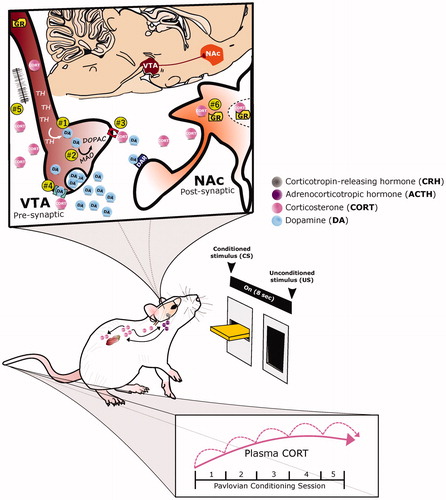
Figure 2. A proposed role for glucocorticoid-dopamine interactions in appetitive conditioning. The integrative response of dopamine (DA) and corticosterone (CORT) under appetitive Pavlovian conditioning is depicted. With repeated pairings of the 8-second presention of a lever-conditioned stimulus (CS) followed by a food-unconditioned stimulus (US), CORT (pink circles) increases both peripherally and centrally, while DA (blue circles) increases within the nucleus accumbens (NAc, orange structure). Corticosterone may potentiate dopamine release by acting within the ventral tegmental area (VTA, maroon structure) to: (1) increase tyrosine hydroxylase (TH), the rate limiting enzyme of DA biosynthesis, or (2) decrease monoamine oxidase (MAO), one of the degradation enzymes of DA. Alternatively, CORT may act presynaptically to mediate DA clearance and/or synaptic uptake by: (3) inhibiting the organic cation transporter (OCT3, red structure) or (4) the dopamine transporter. Finally, CORT may increase DA transmission (5) via glutamatergic synapses or (6) by acting directly upon glucocorticoid receptors (GR) within DA-receptive neurons.