Figures & data
Figure 1. Based on the sucrose preference test, the stressed hedonic (n = 11) and anhedonic rats (n = 16) were identified and compared to nonstressed rats (n = 14) (A). Anhedonia-like behavior was defined as >30% reduction in sucrose consumption compared with baseline (horizontal dotted black line). A significant differences between groups (F(2, 38) = 41.24, p<.0001) and over time (F(4.88, 183.60) = 3.43, p=.006) was seen. The hedonic group did not differ in sucrose index from the non-stressed group, whereas the group with anhedonia-like behavior had a significantly reduced sucrose index after CMS. There was no significant difference in weight changes over time between the groups (B). *p<.01 and ***p<.001 for the anhedonia-like group vs. nonstressed controls; †p < .05, ††p<.01, and †††p<.001 for the anhedonia-like group vs. the hedonic group (ANOVA followed by Tukey correction for multiple comparison).
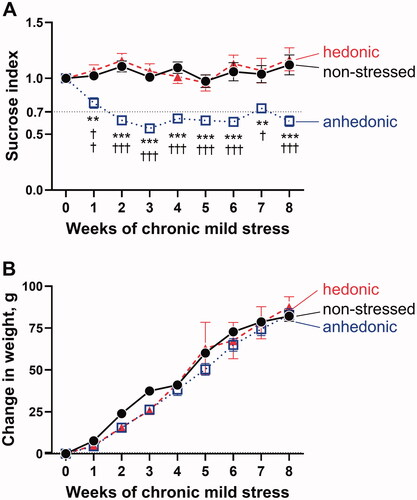
Figure 2. Assessment of neurovascular signaling in brain slices revealed biphasic neurovascular responses in stressed rats with unchanged hedonic state. Representative image of a Calcium Green AM-loaded brain slice from a nonstressed rat shows the neuronal tissue (black tilted arrow) and astrocytic endfood (white tilted arrow) located in a proximity of the adjacent parenchymal arteriole (horizotal arrow indicates arteriole lumen) (A). Brain slices were incubated with 10 nM U46619 for 30 min to pre-constrict parenchymal arterioles to the diameter of approximately 80% of maximally relaxed diameter. Arterioles of the three experimental groups were similar in diameter after pre-constriction with U46619 (B). The fully relaxed arteriole diameter measured in Ca2+-free artificial cerebral spinal fluid was also similar in all the three groups (C). Electric field stimulation (EFS, indicated with vertical dashed lines) of the neuronal tissue in brain slices was associated with vasodilation of parenchymal arterioles in nonstressed (n = 6), hedonic (n = 5) and anhedonic rats (n = 7) (D). In hedonic rats, the initial vasodilation was followed by vasoconstriction. Superfusion of the brain slices with 100 µM BaCl2 strongly diminished neurovascular responses in all groups, but the subsequent vasoconstriction was still observed in hedonic group (E). There was no difference in BaCl2-sensitive vasodilation among the three experimental groups (F). All the three groups showed similar increase in intracellular Ca2 in the neuronal tissue (G) and in astrocytic endfeet (H) in response to EFS. Two nonstressed brain slices and three slices from the anhedonia-like rats were excluded from Ca2+ astrocytic signaling analyses as no astrocytes were identified in the field of view. The data in (B), (C), and (F) were compared with one-way ANOVA; the data in (D), (E), (G), and (H) were compared with two-way ANOVA. **p < .01 and ***p<.001, respectively.
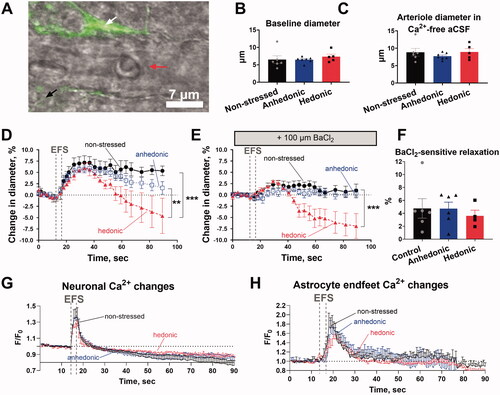
Figure 3. Middle cerebral arteries from stressed rats with unchanged hedonic state exhibited increased arterial contractility. Middle cerebral arteries from hedonic (n = 6) and anhedonic (n = 9) rats showed increased constriction to U46619 compared with nonstressed control rats (n = 8) (A). Middle cerebral arteries pre-constricted with 10 nM U46619 from nonstressed (n = 6), hedonic (n = 9), and anhedonic groups (n = 6) relaxed in response to the elevation of bath K+ concentration up to 18 mM (B). At 20 mM and 24 mM of bath K+, the arteries from hedonic rats showed a decline in vasorelaxation, whereas arteries from anhedonic and nonstressed rats remained relaxed. The K+-induced relaxation was inhibited by 100 µM BaCl2; but at 24 mM bath K+, arteries from hedonic rats constricted (C). The concentration–response curves in (A) were compared with an extra sum-of-squares F test; the responses in (B) and (C) were analyzed with two-way ANOVA followed by Tukey correction for multiple comparison. *p < .05 and **p< .01 for nonstressed vs. hedonic rats; †p < .05 for anhedonia-like vs. nonstressed rats; +p < .05 for anhedonia-like vs. hedonic rats.
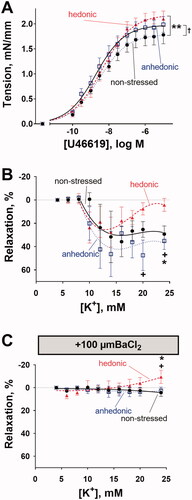
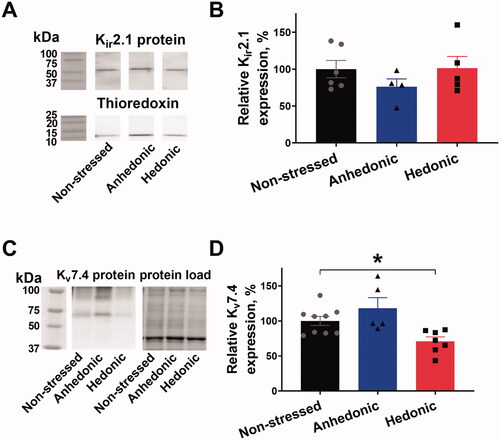
Figure 4. Cerebral arteries from stressed rats with unchanged hedonic state had reduced expression of Kv7.4 channels but unchanged Kir2.1 expression. Lysate of middle cerebral arteries was blotted for the Kir2.1 channel expression with thioredoxin as a loading control. Representative blots of Kir2.1 and thioredoxin from the same lanes (A); the vertical white space between the bands indicates that irrelevant lanes from the same film are not shown. No difference in relative Kir2.1 expression was seen between middle cerebral arteries from nonstressed (n = 6), hedonic (n = 4), and anhedonic rats (n = 5) (B). Representative blots for Kv7.4 protein detection and total protein load as identified with stain-free gel loading control. The expression of Kv7.4 protein was reduced in arteries from hedonic rats (n = 7) in comparison with nonstressed rats (n = 9). *p < .05 for nonstressed vs. resilient rats (one-way ANOVA followed by Dunnett correction for multiple comparison).
Cerebral arteries from stressed rats with unchanged hedonic state had reduced expression of Kv7.4 channels but unchanged Kir2.1 expression.