Figures & data
Figure 1 Increased Ang II receptor expression in the PVN after repeated restraint stress. The figure represents a typical autoradiography image of Ang II receptor binding in a coronal section of the hypothalamus through the paraventricular nuclei, as determined after incubation in the presence of [125I]-Sarcosine1-Ang II, in control rats and in rats submitted to seven repeated sessions of 2 h restraint. Black arrow indicates the third ventricle. Binding is concentrated in the parvocellular region of the PVN (white arrow). Note the intense signal generated after repeated restraint stress. Bar is 0.5 mm (modified from Castrén and Saavedra Citation1988).
![Figure 1 Increased Ang II receptor expression in the PVN after repeated restraint stress. The figure represents a typical autoradiography image of Ang II receptor binding in a coronal section of the hypothalamus through the paraventricular nuclei, as determined after incubation in the presence of [125I]-Sarcosine1-Ang II, in control rats and in rats submitted to seven repeated sessions of 2 h restraint. Black arrow indicates the third ventricle. Binding is concentrated in the parvocellular region of the PVN (white arrow). Note the intense signal generated after repeated restraint stress. Bar is 0.5 mm (modified from Castrén and Saavedra Citation1988).](/cms/asset/3c45eee0-d8c1-427f-88e4-2df9bd4aa5b9/ists_a_234965_f0001_b.gif)
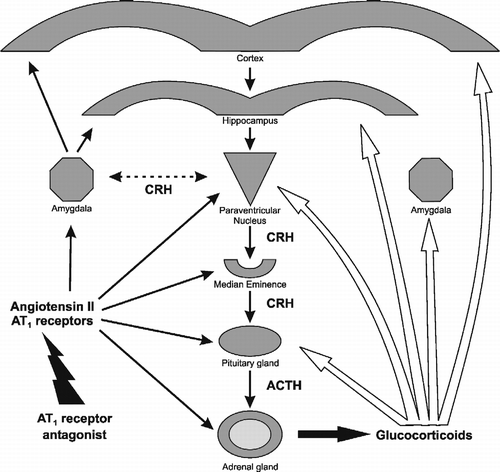
Figure 2 Effect of pretreatment with an AT1 receptor antagonist on the stress-induced pituitary ACTH content and urinary corticosterone excretion. Isolation stress increases pituitary ACTH. Pretreatment with candesartan prevents the increase in pituitary ACTH and decreases the urinary excretion of corticosterone. *P < 0.05 vs. grouped control and isolated rats pretreated with candesartan. #P < 0.05 vs. isolated rats treated with vehicle (modified from Armando et al. Citation2001).
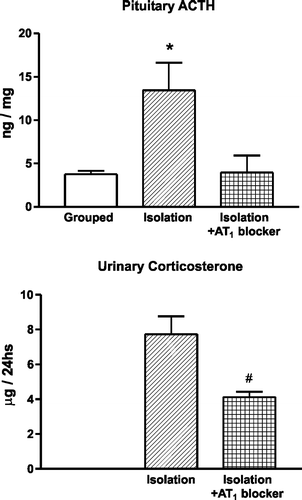
Figure 3 Prevention of gastric mucosal lesions induced by cold restraint stress by pretreatment with AT1 receptor antagonists. Microphotographs of hematoxylin-eosin-stained sections of the glandular portion of the stomach in rats submitted to cold-restraint stress treated with vehicle (left) or with candesartan (right), for 2 weeks before the stress. The lesion in the rat submitted to stress and treated with vehicle (left) involves the entire depth of the gastric mucosa. Pretreatment with candesartan for 2 weeks (right) prevented the development of stress-induced gastric ulcers. Bar is 100 μm (modified from Bregonzio et al. Citation2003).
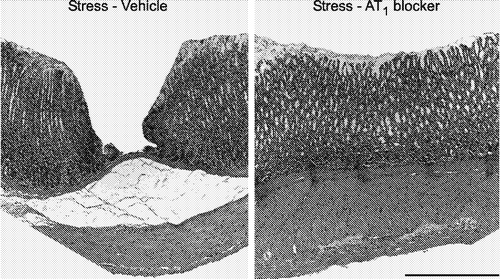
Figure 4 Effect of AT1 receptor blockade on expression of CRF1 receptor binding in the cerebral cortex of rats submitted to isolation stress. Figures represent autoradiographic images of cortical sections incubated in the presence of 0.2 nM of [125I]-sauvagine to reveal CRF receptors. Arrows point to cortex, layer IV. Bar is 3 mm. Columns are quantitative autoradiographic measurements of CRF1 receptor binding, specifically displaceable by addition of 13 nM antalarmin. Results are means ± SEM, for groups of six rats measured individually. *P < 0.05 vs. control grouped and isolated, candesartan-treated rats, one-way ANOVA followed by Neuman Keul's test (modified from Saavedra et al. Citation2006).
![Figure 4 Effect of AT1 receptor blockade on expression of CRF1 receptor binding in the cerebral cortex of rats submitted to isolation stress. Figures represent autoradiographic images of cortical sections incubated in the presence of 0.2 nM of [125I]-sauvagine to reveal CRF receptors. Arrows point to cortex, layer IV. Bar is 3 mm. Columns are quantitative autoradiographic measurements of CRF1 receptor binding, specifically displaceable by addition of 13 nM antalarmin. Results are means ± SEM, for groups of six rats measured individually. *P < 0.05 vs. control grouped and isolated, candesartan-treated rats, one-way ANOVA followed by Neuman Keul's test (modified from Saavedra et al. Citation2006).](/cms/asset/80e0f9d0-06c6-423c-ba2f-25a6b47f0940/ists_a_234965_f0004_b.gif)
Figure 5 Anxiolytic effect of candesartan. The figure shows the increase in the time spent in the open arms, the number of open arm entries and the number of closed arm entries in an elevated plus-maze by rats pretreated for 14 days with 0.5 mg/kg/day of candesartan (AT1 blocker), administered subcutaneously by means of an osmotic minipump. Increased number of open arm entries and increased time spent in the open arm are signs of decreased anxiety. The total time on the plus-maze was 5 min. *P < 0.05 vs. vehicle-treated rats (modified from Saavedra et al. Citation2006).
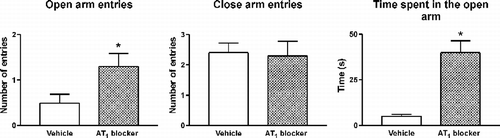
Figure 6 Sites of effect of AT1 receptor antagonists on the regulation of peripheral and brain components of the stress reaction. Left black arrows: AT1 receptor antagonists block AT1 receptor stimulation in the adrenal and pituitary glands, the median eminence, PVN, amygdala, hippocampus and cerebral cortex. Right white arrows: sites of glucocorticoid feedback regulation during stress in the pituitary gland, the median eminence, PVN, hippocampus and cortex.