Figures & data
Figure 1 Diagram depicting the sequence of events in the regulation of CRF expression in the PVN. Following release of stimulatory neurotransmitters in the PVN in response to stress, there is a rapid release of CRF peptide from the median eminence into the pituitary portal circulation, as evidenced by a rapid disappearance of immunoreactive CRH from the median eminence (I). The image shows immunostaining for CRF in the median eminence of rats subjected to intracerebroventricular colchicine injection 18 h before ip injection of normal saline (controls) or HS (stress). This is followed by transient increases in CRF primary transcript (hnRNA) within minutes (II), and more sustained increases in CRF mRNA, within hours (III), shown by the PVN in situ hybridization images and time curves. Restoration of CRF peptide storage pools through mRNA translation occurs within hours or days (IV).
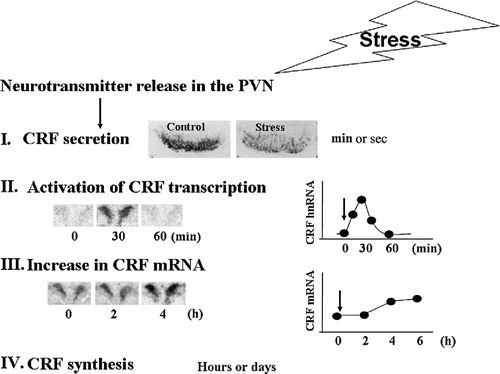
Figure 2 Adrenalectomy (ADX) sensitizes CRF hnRNA responses to the minor stressor of ip injection of 100 μl of 50% ethanol in normal saline (used as vehicle for corticosterone (Cort) injections). (A) Time course of the changes in plasma corticosterone levels following injection of corticosterone (2.8 mg/100 g BW, ip) or vehicle (Veh) in 48-h adrenalectomized or sham-operated rats. Data points represent the mean and SE of values obtained in six rats per experimental group. *, p < 0.01 vs. ADX or sham basal (0 min). (B) Time course of the changes in CRF hnRNA after injection of corticosterone (2.8 mg/100 g BW, ip) or vehicle in 48-h adrenalectomized or sham-operated rats. Data points are the mean and SE of the optical density values obtained from film autoradiograms in six rats per experimental group. *, p < 0.01 vs. sham or ADX basal (0 min). From Ma and Aguilera (Citation1999a).
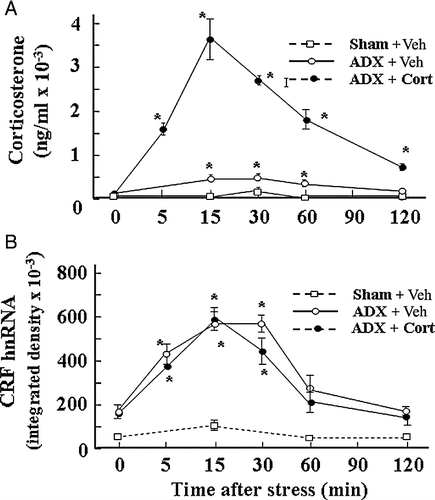
Figure 3 Effect of glucocorticoids on plasma corticosterone levels and CRF hnRNA responses to the painful stress of ip HS injection in intact rats. Time course of the changes in plasma corticosterone levels (A) or CRF hnRNA in the PVN (B), following administration of corticosterone (2.8 mg/100 g BW, ip) injected either at − 30 min or at 0 min before ip HS injection. Data points represent the mean and SE of values obtained in six rats per experimental group. *, p < 0.01 vs. ADX or sham basal (0 min). (B) Time course of the changes in CRF hnRNA after injection of corticosterone (2.8 mg/100 g BW, ip) or vehicle in 48-h adrenalectomized (ADX) or sham-operated rats. Data points are the mean and SE of the optical density values obtained from film autoradiograms in six rats per experimental group. *, p < 0.01 vs. sham or ADX basal (0 min).
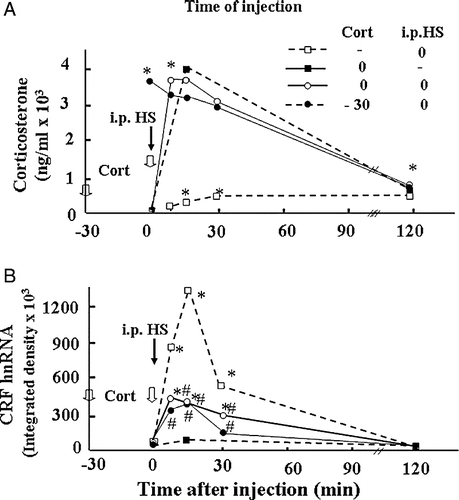
Figure 4 Glucocorticoids do not mediate the rapid decline of CRF gene transcription during stress. Plasma corticosterone levels (A) and CRF hnRNA levels in the PVN (B) during the course of 3 h restraint stress in sham-operated and adrenalectomized (ADX) rats receiving constant levels of corticosterone (Cort) via subcutaneous implants. Rats were killed by decapitation at the indicated time points. Data points are the mean and SE of the values obtained in five rats per experimental group. * p < 0.001 compared to basal; # p < 0.05 compared to basal sham-operated rats. From Shepard et al. (Citation2005).
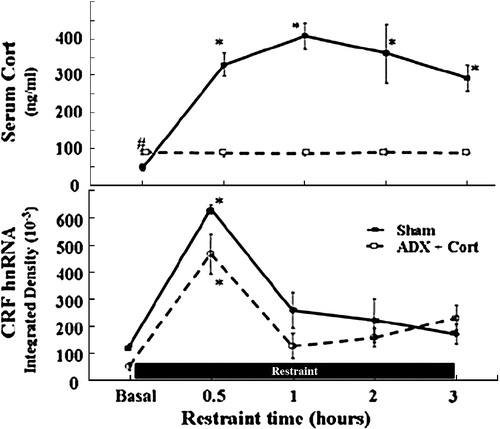
Figure 5 Stress increases CREM mRNA levels in the PVN. (A) Changes in CREM mRNA measured by in situ hybridization using 35S-labeled cRNA probes. Data points are the mean and SE of the pooled data. *, p < 0.001 compared with basal. Representative images from film autoradiography of 1 of 5 rats per experimental group are shown under the respective time points. Almost identical results were shown using a 48-mer oligoprobe specific for ICER. (B) Cellular localization of CREM mRNA in PVN sections of rats subjected to restraint stress for 3 h. Double labeling in situ hybridization using 35S-labeled CREM cRNA probes (bright green grains), and digoxigenin-labeled CRF cRNA probes, shows 35S-CREM staining (bright grains) overlaying digoxigenin-labeled parvicellular CRF neurons. Third V, 3rd ventricle; the lower panel shows higher magnification of the area enclosed in the square. The white arrowheads indicate some of the CRF neurons labeled with CREM. CREM staining in control non-stressed rats was no different from background staining (12.3 ± 1.8 and 9.6 ± 1.4 grains/cell, not shown). Adapted from Shepard et al. (Citation2005).
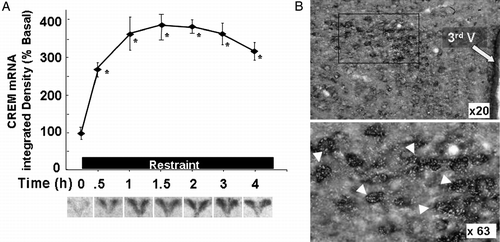
Figure 6 Restraint stress induces recruitment of phospho-CREB and CREM by the CRF promoter in the hypothalamic PVN region. (A) Chromatin immunoprecipitation (ChIP) assays using phospho-CREB, CREM and Pol II antibodies and cross-linked DNA from microdissected hypothalamic PVN region of control rats and rats subjected to restraint stress for 30 min or 3 h. (B) PCR for the CRF coding region, performed on the same immunoprecipitants used in (A), showed non-specific bands, which were not changed by stress. Gel images are representative of the results in three experiments. Ab, antibody; pCREB, phospho-CREB; Pol II, RNA polymerase II. Adapted from Shepard et al. (Citation2005).
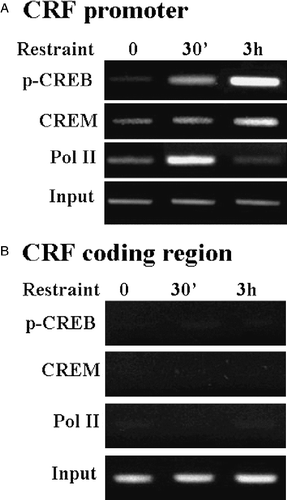
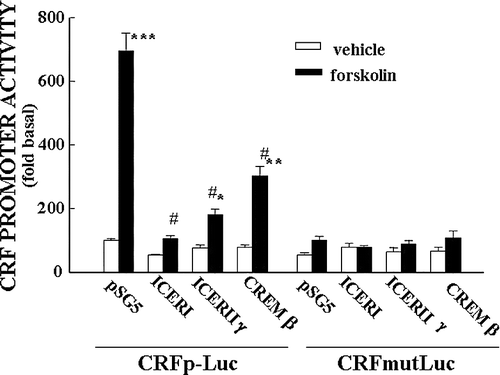
Figure 7 Repressor isoforms of CREM inhibit CRF promoter activity in a CRE dependent manner. Effect of co-transfection of the expression vector, pSG5, empty or containing ICER I, ICER II or CREMβ on basal and forskolin-stimulated CRF promoter activity in the hypothalamic cell line, H32 simultaneously transfected with CRF promoter–luciferase reporter genes. Cells were co-transfected with CRF promoter–luciferase reporter plasmids, without (CRFp-Luc) or with inactivating mutations of the CRE (CRFmut-Luc) and the expression vector, and 18 h later incubated with forskolin for 6 h before measurement of luciferase activity. Bars represent the mean and SE of the data obtained in three experiments. * p < 0.01 vs. respective vehicle; #, p < 0.01 compared to forskolin-stimulated pSG5. From Liu et al. (Citation2006).