Figures & data
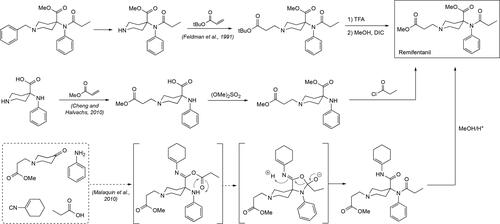
Figure 1. Structure of fentanyl and the analogs discussed in this review along with the morphine and the pethidine scaffolds. A detailed and large medicinal chemistry approach based on the pethidine nucleus gave birth to the fentanyl class of opioids. In addition, the structures of the two most common, current antidotes to treat fentanyl overdose, Naloxone and Naltrexone, are given. Where applicable for the fentanyls, other common names as well as tradenames are provided along with their individual potencies relative to morphine are given in brackets.
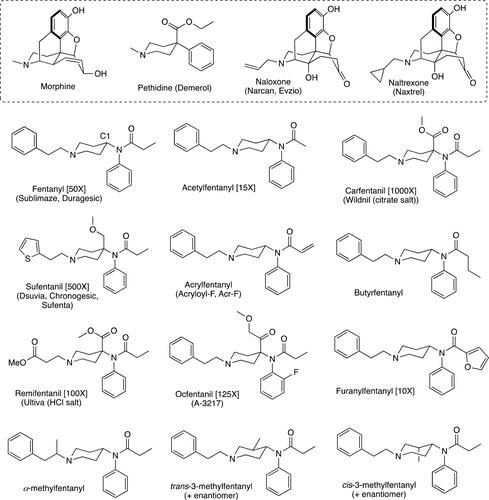
Figure 2. General protocol for sample preparation for the GC-MS analysis of fentanyl and related opioids in biological and environmental* samples. *By environmental it is meant that the sample originated from the environment where the fentanyl was collected and this may include wipe samples from surfaces or washed samples from objects associated with an overdose case. EtOAc = ethyl acetate; MeOH = methanol.
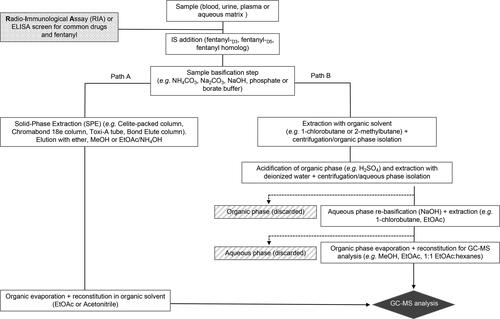
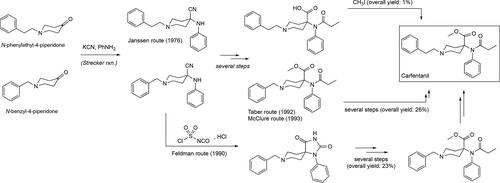
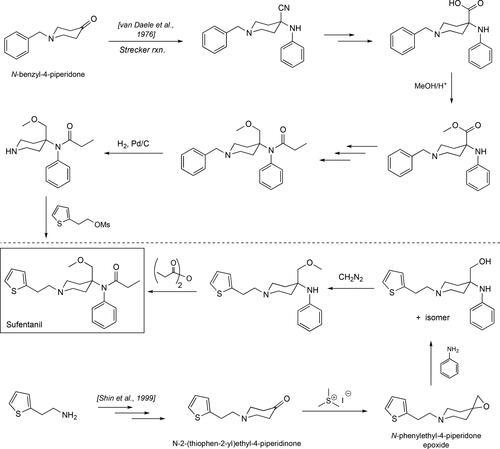
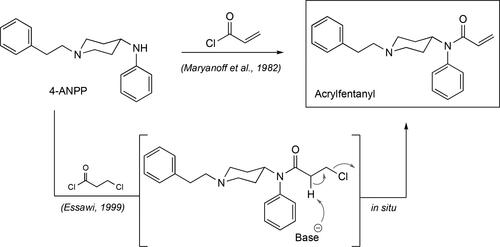
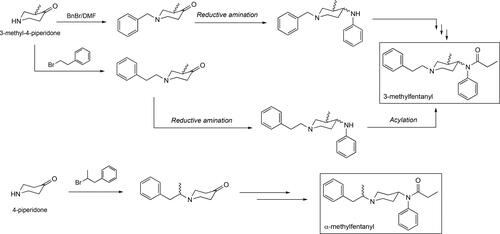
Figure 3. Some of the synthetic pathways to fentanyl including the original one published by Janssen. Each arrow in the scheme represents a step in the synthetic route. Janssen’s route makes use of the benzylated piperidone material and its presence adds two additional steps, debenzylation followed by alkylation with (2-chloroethyl)benzene, relative to the Siegfried method[Citation65] and the one published by Valdez et al.[Citation36]
![Figure 3. Some of the synthetic pathways to fentanyl including the original one published by Janssen. Each arrow in the scheme represents a step in the synthetic route. Janssen’s route makes use of the benzylated piperidone material and its presence adds two additional steps, debenzylation followed by alkylation with (2-chloroethyl)benzene, relative to the Siegfried method[Citation65] and the one published by Valdez et al.[Citation36]](/cms/asset/d4e3a183-add6-4916-a22e-f0f663325a86/batc_a_1927668_f0003_b.jpg)
Table 1. Summary of discussed GC-MS analyses for fentanyl.
Table 2. Summary of discussed GC-MS analyses for acetylfentanyl.
Table 3. Highlighted list of GC-MS analyses of other fentanyls discussed in this review.
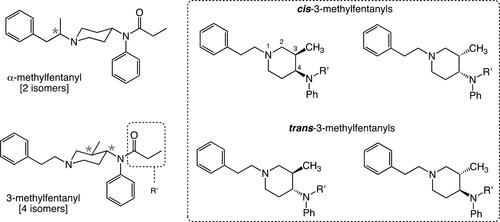
Figure 8. Chemical structures of ?-methylfentanyl and 3-methylfentanyl. The asterisks in each structure denote a chiral carbon center. In ?-methylfentanyl the presence of one chiral center gives rise to the existence of two stereoisomers, while the presence of two chiral centers in 3-methylfentanyl gives rise to the existence of four isomers (a pair of enantiomers and a pair of diastereomers).