Figures & data
Figure 1. Chemical structure of vitamin D2 (A), vitamin D3 (B), and vitamin D4 (C). In online version, differences are shown in blue.
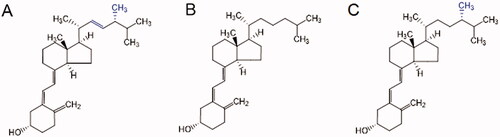
Figure 2. Synthesis of vitamin D. Upon UV-B radiation, the provitamins D ergosterol, 7-dehydrocholesterol, and 22,23-dihydroergosterol are respectively converted to pre-vitamins D2, D3, and D4, which are further thermally transformed into ergocalciferol (vitamin D2), cholecalciferol (vitamin D3), and 22,23-dihydroergocalciferol (vitamin D4). Only structures of provitamin D3, pre-vitamin D3, and vitamin D3 are depicted for better lucidity.
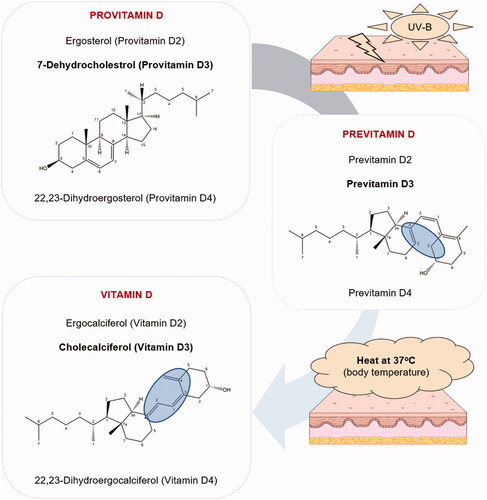
Table 1. Vitamin D content in selected foodstuff and preparations.
Figure 3. Vitamin D absorption. Hydroxylated forms of vitamin D are absorbed directly into the vena portae by an unknown mechanism (1). Intact vitamin D is first built into mixed micelles (2). The uptake of vitamin D at dietary concentrations is protein-mediated (3), while at higher pharmacological concentrations it is absorbed through passive diffusion as well (4). Chylomicrons containing vitamin D are then secreted into the lymphatic capillaries (5) before reaching systemic circulation (6). C: cluster determinant 36 (CD36); D: vitamin D; GIT: gastrointestinal; N: Niemann-Pick C1-Like 1 (NPC1L1); S: scavenger receptor class B type 1 (SR-B1).
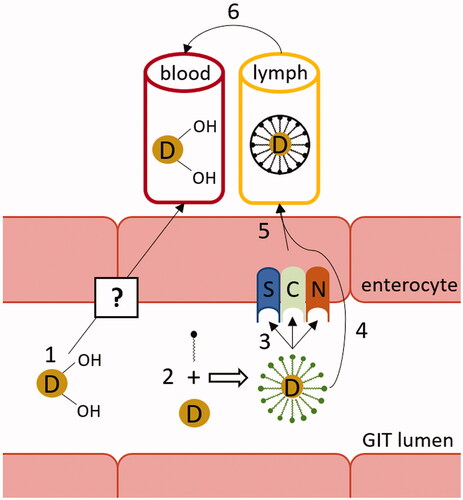
Figure 4. Systemic vitamin D3 metabolism and its regulation. FGF23: fibroblast growth factor 23; PTH: parathyroid hormone.
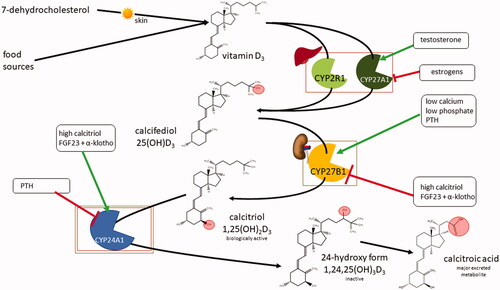
Figure 5. Summary of genetic disorders in vitamin D3 metabolism. Vitamin D-dependent rickets (VDDR) type 1 is caused by the hypofunction of the activating enzyme CYP27B1. VDDR type 1 b arises one step earlier due to the hypofunction of the activating enzyme CYP2R1. VDDR type 2, also called vitamin D-resistant rickets, is caused by dysfunction of the substrate recognition site of the vitamin D receptor (VDR). VDDR type 3 is caused by the gain-of-function mutation of CYP3A4, which starts to deactivate vitamin D metabolites with even greater activity than CYP24A1.
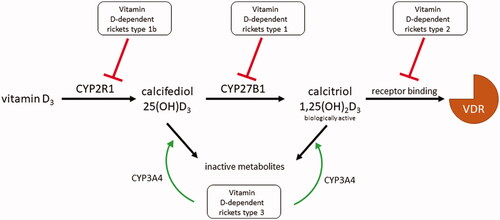
Table 2. Types of rickets induced by alterations in vitamin D metabolism.
Figure 6. Mechanism of vitamin D (calcitriol) action. In this figure vitamin D should be understood as its active form - calcitriol. The majority of circulating vitamin D is bound to vitamin D binding protein (vDBP) (1). This complex may only enter cells with the megalin/cubulin system (LRP2) (2). Free vitamin D can enter any cell through passive diffusion (3). vDBP-bound vitamin D is released inside the cells (4). In the cytoplasm, vitamin D interacts with its receptor (VDR) and creates a heterodimer with retinoid X receptor (RXR) (5). The active VDR complex enters the nucleus (6) and binds to the responsive elements (VDRE) of regulated genes.
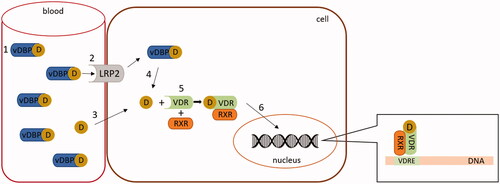
Figure 7. Calcium absorption in the enterocyte. In hypocalcemia, calcitriol upregulates calcium transient receptor potential vanilloid channel 6 (TRPV6) and calbindin-D9k in the enterocyte, thus stimulating the calcium absorption in the intestine. Also, the expression of plasma membrane calcium pump type 1b (PMCA1b) is increased by calcitriol. GIT: gastrointestinal tract.
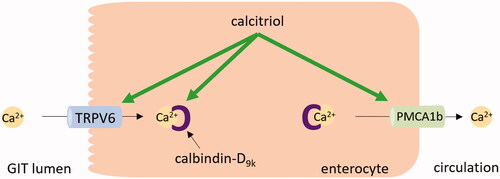
Figure 8. Vitamin D overdose. Excessive intake of vitamin D due to intentional or inadvertent incorrect dosing (e.g. prescribing errors, supplementation with products that have low or no quality control) may increase plasma 25-hydroxyvitamin D [25(OH)D] to concentrations susceptible to causing toxicity. NOAEL: no observed adverse effect level; UL: tolerable upper intake level.
![Figure 8. Vitamin D overdose. Excessive intake of vitamin D due to intentional or inadvertent incorrect dosing (e.g. prescribing errors, supplementation with products that have low or no quality control) may increase plasma 25-hydroxyvitamin D [25(OH)D] to concentrations susceptible to causing toxicity. NOAEL: no observed adverse effect level; UL: tolerable upper intake level.](/cms/asset/546aa3cb-ca08-41ef-9f4e-e64abcac4cb9/ilab_a_2070595_f0008_c.jpg)
Figure 10. Theories proposed by Jones et al. [Citation307] for the mechanisms of toxicity of vitamin D. The first mechanism proposed for explaining vitamin D toxicity involves a plasma increase in calcitriol [1,25(OH)2D]. This active form of vitamin D has low affinity to the vitamin D binding protein (vDBP) and high affinity to the vitamin D receptor (VDR), leading to a critical increase in calcitriol in the target cells and subsequent overstimulation of the gene expression machinery. A second theory proposes an increase in plasma vitamin D metabolites to concentrations that saturate vDBP, allowing high free levels of these metabolites to enter the target cells, in particular 25-hydroxyvitamin D [25(OH)D] that has a greater affinity to VDR. The last mechanism is related to the release of calcitriol from vDBP because it has the lowest affinity for this plasma protein compared to other vitamin D metabolites. 24,25(OH)2D: 24,25-dihydroxyvitamin D; 25,26(OH)2D: 25,26-dihydroxyvitamin D; 25(OH)D-26,23-lactone: 25-hydroxyvitamin D-26,23-lactone.
![Figure 10. Theories proposed by Jones et al. [Citation307] for the mechanisms of toxicity of vitamin D. The first mechanism proposed for explaining vitamin D toxicity involves a plasma increase in calcitriol [1,25(OH)2D]. This active form of vitamin D has low affinity to the vitamin D binding protein (vDBP) and high affinity to the vitamin D receptor (VDR), leading to a critical increase in calcitriol in the target cells and subsequent overstimulation of the gene expression machinery. A second theory proposes an increase in plasma vitamin D metabolites to concentrations that saturate vDBP, allowing high free levels of these metabolites to enter the target cells, in particular 25-hydroxyvitamin D [25(OH)D] that has a greater affinity to VDR. The last mechanism is related to the release of calcitriol from vDBP because it has the lowest affinity for this plasma protein compared to other vitamin D metabolites. 24,25(OH)2D: 24,25-dihydroxyvitamin D; 25,26(OH)2D: 25,26-dihydroxyvitamin D; 25(OH)D-26,23-lactone: 25-hydroxyvitamin D-26,23-lactone.](/cms/asset/ca9b824d-0752-4516-a14e-4c16d5177d56/ilab_a_2070595_f0010_c.jpg)
Table 3. Cases of vitamin D toxicity reported in the literature.
Table 4. Summary of the main benefits and limitations of the different immunoassays and chromatographic approaches to analyze vitamin D and its metabolitesa.