Figures & data
Table 1. Studies comparing the OSM between patients with DED and healthy people using 16S rRNA gene sequencing.
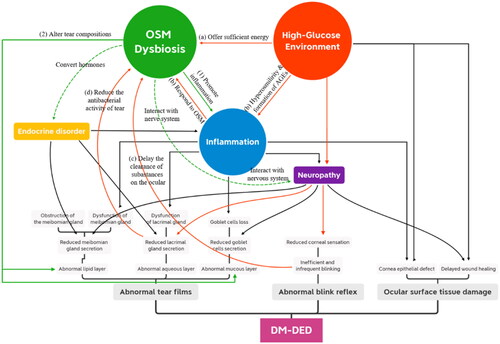
Figure 1. The “high glucose-OSM dysbiosis” pathway in the pathogenesis of DM-DED. Red lines refer to how a high-glucose environment leads to OSM dysbiosis, while green solid lines refer to how OSM dysbiosis contributes to the pathogenesis of DM-DED. Green dotted lines refer to the possible mechanisms that remain to be investigated. The high-glucose ocular surface environment leads to OSM dysbiosis in DM-DED through multiple pathways. (a) High glucose content offers sufficient energy for microbial growth. (b) Hyperosmolarity and the formation of AGEs induce ocular inflammation, which leads to massive microbial death. (c) Reduced blinking frequency delays the clearance of substances on the ocular surface. (d) Lacrimal gland dysfunction causes the reduced antibacterial activity of tears. Then, OSM dysbiosis (1) promotes ocular surface inflammation and (2) alters tear composition, which contributes to the pathogenesis of DM-DED. In addition, the OSM may also influence the homeostasis of the ocular surface by interacting with the ocular surface nervous system and converting hormones.