Figures & data
Figure 1. A positive feedback cycle in the pathogenesis of periodontitis. Pathobionts colonise subgingival niches, subvert host immune responses and proliferate, which leads to dysbiosis in the microbial community. The dysbiotic microbial community, characterised by overgrowth of a small number of pathobionts and overproduction of bacterial toxins, persistently stimulates local inflammatory responses. Inflammatory tissue breakdown in turn produces nutrients, including peptides, haem and ferrous iron, that further facilitate the proliferation of pathobionts. This also results in periodontal pockets deepening providing more suitable habitat for pathobionts. Collectively, the dysbiotic microbiota and inflammation share a mutually supportive relationship which is presented as a positive feedback cycle in the development of periodontitis.
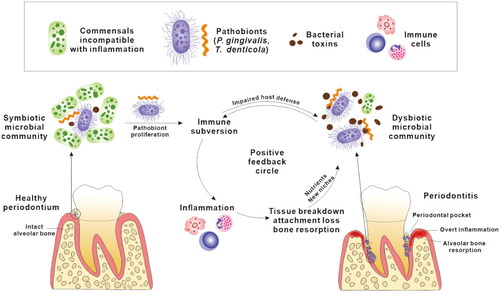
Figure 2. Current proposals on how oral bacteria may promote the progression of Alzheimer’s disease. Oral bacteria have been proposed to promote AD pathology by two mechanisms. Firstly, oral bacteria may impact the central nervous system indirectly by causing periodontitis, by aggravating pulmonary diseases after aspiration to the respiratory system and by provoking microbiota-gut-brain axis disorders in the alimentary tract (Wu et al. Citation2017; Xue et al. Citation2020). This induces chronic systemic inflammation which subsequently extends to the brain adding to the load of neuroinflammation. Secondly, discontinuous sites within inflamed periodontal pocket epithelia allow periodontal bacteria to invade adjacent primary afferent nerves and blood vessels, along which they escape the oral cavity and directly access the brain, leading to brain infection.
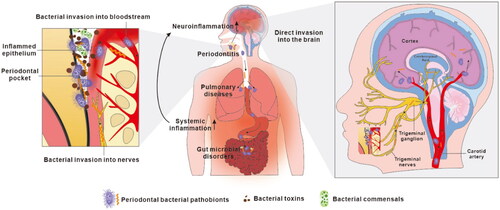
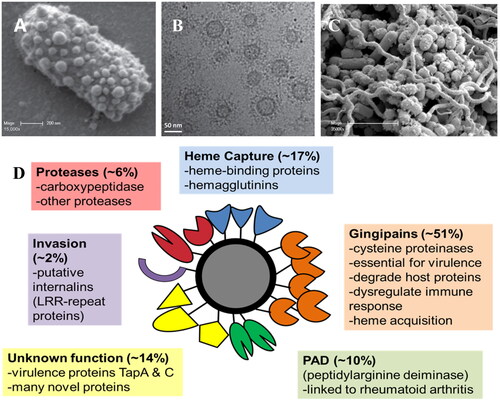
Figure 3. (A) Scanning electron micrograph showing the high numbers of OMVs blebbing from the surface of a P. gingivalis cell. (B) Cryo-transmission electron micrograph showing P. gingivalis OMVs and their extensive surface layer of proteins (reproduced with permission from Gui et al. (Citation2016)). (C). Scanning electron micrograph of a pathogenic polymicrobial biofilm of P. gingivalis, T. denticola and T. forsythia showing high numbers of OMVs (reproduced with permission from Zhu et al. (Citation2013)). (D) The P. gingivalis OMV proteome is composed largely of Type IX Secretion System proteins that play roles in host tissue destruction, immune response dysregulation, internalisation and (micro)nutrient capture.