Figures & data
Figure 1. Overview lipid and carbohydrate uptake and catabolism. There are different routes for uptake and catabolism of lipids and carbohydrates within mtb. Starting molecules for each pathway are cholesterol, FAs, and glucose (red text). The different pathways contain proteins (italic) or metabolic intermediates (black text). Proteins were found to be essential for survival of mtb in vitro or in vivo (green text), while others are conditionally essential when grown on a specific nutrient source (orange text) (Sassetti and Rubin Citation2003; Griffin et al. Citation2011; DeJesus et al. Citation2017; Beites et al. Citation2021). The depicted pathways can be continuous (black arrow) or shown partially (discontinuous arrow). Pathways occur either in the macrophage phagosome or inside the bacteria. ‘?’ responsible protein not (yet) identified. Created with Bio-Render.com.
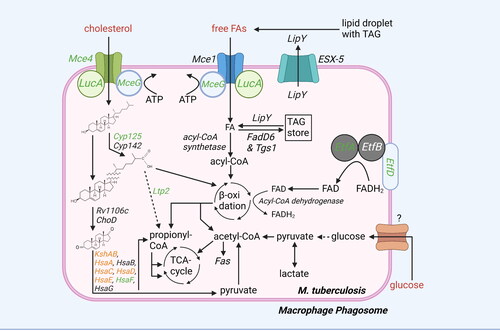
Figure 2. Overview glycolysis, gluconeogenesis, TCA cycle, MCC cycle, and MMC cycle. Mtb uses different pathways to generate ATP, produce molecules for lipid biosynthesis, or detoxify propionyl-CoA. Potential starting molecules for the different pathways are shown (red text). The different pathways contain proteins (italic) or metabolic intermediates (normal text). Proteins were found to be essential for survival of mtb in vitro or in vivo (green text), while others are conditionally essential when grown on a specific nutrient source (orange text) (Sassetti and Rubin Citation2003; Puckett et al. Citation2014Basu et al., 2018; Ganapathy et al. Citation2015; Gutka et al. Citation2015; DeJesus et al. Citation2017). Furthermore, the glyoxylate shunt, not present in mammals, which can be used for gluconeogenesis is shown (blue arrows). TCA cycle intermediates are also shown. Created with Bio-Render.com.
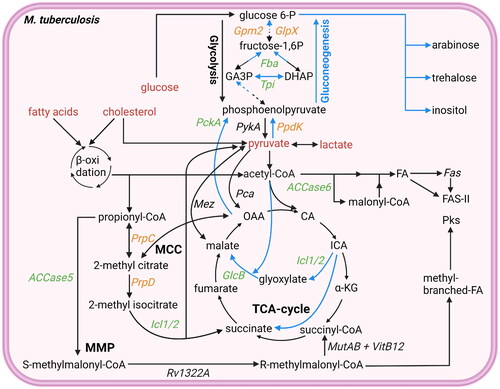
Figure 3. Overview lipid anabolism and production of lipid-based virulence factors: besides being used for ATP production, acetyl-CoA and propionyl-CoA are also used in lipid anabolism and virulence factor production. Potential starting molecules for the different pathways are shown (red text). The different pathways contain proteins (italic) or metabolic intermediates (normal text). Proteins were found to be essential for survival of mtb in vitro or in vivo (green text), while others are conditionally essential when grown on a specific nutrient source (orange text) (Sassetti and Rubin Citation2003; Kalscheuer et al. Citation2010; Varela et al. Citation2012; Bazet Lyonnet et al. Citation2014; DeJesus et al. Citation2017; Fay et al. Citation2019; Pohane et al. Citation2021). The depicted pathways can be continuous (black arrow) or shown partially (discontinuous arrow). Pathways occur either in the cytosol or on the outer membrane of the bacteria (outside the cytosol). Created with Bio-Render.com.
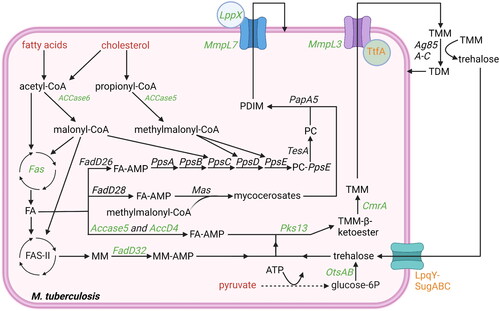
Figure 4. Overview carbohydrate pathways and production of carbohydrate-based virulence factors. To modulate host cells to deliver lipid nutrients, mtb requires carbohydrate-based virulence factors. Carbohydrate products can be derived from lipid nutrients in glucose-deprived conditions (). Potential starting molecules for the different pathways are shown (red text). The different pathways contain proteins (italic) or metabolic intermediates (normal text). Proteins were found to be essential for survival of mtb in vitro or in vivo (green text), while others are conditionally essential when grown on a specific nutrient source (orange text) (Jackson et al. Citation2000; Sassetti and Rubin Citation2003; Movahedzadeh et al. Citation2004; Kaur et al. Citation2007; Guerin et al. Citation2009; Movahedzadeh et al. Citation2010; Boldrin et al. Citation2014; DeJesus et al. Citation2017; Boldrin et al. Citation2021). The depicted pathways can be continuous (black arrow) or shown partially (discontinuous arrow). PPM (blue) and PP (purple) generated via the pentose-6P pathways are used both to elongate LAM and LM, as well as generate Ac2PIM6 from Ac2PIM6. The biosynthesis of PIMs starts on the cytosolic side of the plasma membrane, but from PIM4 onwards, the production occurs on the periplasmic side. ‘?’ responsible protein not identified. Created with Bio-Render.com.
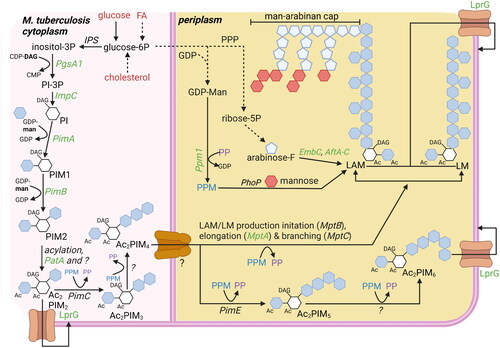
Figure 5. Pathogen host interactions during mtb infections to sequester nutrients and promote intracellular survival. Upon recognition of mtb by TLRs, mtb is phagocytosed into macrophages. Once inside the macrophage, mtb exploits multiple mechanisms to survive intracellularly and to acquire nutrients. Mtb uses the virulence factors PDIM and ESAT6 to induce membrane permeabilisation of the phagosome to escape from the phagosome. Cytosolic mtb can migrate toward cytosolic LDs inside the macrophage. Alternatively, mtb can be recognized by the host and returned to a phagosome via xenophagy. However, potentially by stimulating TLR4 ligation, mtb can induce lipophagy of cytosolic LDs. As lipophagy and xenophagy use similar pathways, vacuoles containing both mtb and LDs can form, thereby representing a mechanism how mtb could also stimulate migration of nutrients toward itself. Once in a vacuole with LDs, mtb utilizes multiple lipid-based virulence factors to prevent phagosomal maturation and thereby protects itself from degradation. Additionally, mtb can stimulate the uptake of lipids by macrophages to increase the nutrient source available. To do so, mtb induces TLR2 ligation to stimulate mTORC1 signaling, which induces PPAR-γ expression. PPAR-γ is then thought to induce uptake of LDLs and FAs via endocytosis. The endocytosed lipids are then stored in cytosolic LDs in close proximity to mitochondria, thereby increasing the nutrient availability that mtb can acquire via the mechanisms described above. TLR2, blue; TLR4, green. Created with Bio-Render.com.
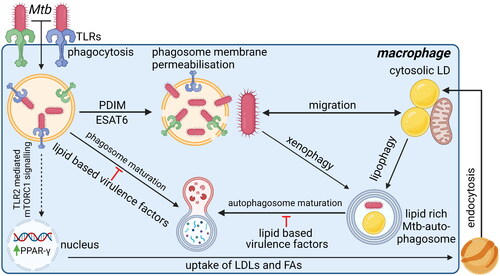
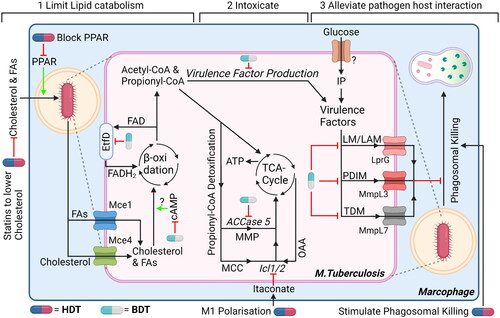
Figure 6. Three suggestions for dual-acting therapies that target both mtb and host cells to facilitate synergistic therapies. (1) Impairing bacterial cholesterol metabolism by targeting bacteria-specific EtfD to impair β-oxidation or RV1625c to promote upregulation of bacterial cAMP, which impairs cholesterol metabolism. In addition, limit availability of cholesterol (and FAs) in host cells using statins and/or PPAR. (2) Promote intoxication by propionyl-CoA, which is produced as by-product during β-oxidation, by blocking detoxification pathways using ACCase5 and Icl1/2 inhibitors. Host itaconate will further inhibit Icl1/2, whose levels can be increased by promoting host macrophages polarization toward M1-like phenotype or administering endogenous itaconate. (3) Alleviating host-pathogen interaction will promote intracellular degradation of mtb. Inhibiting bacterial transporters MmpL7, MmpL3 and/or LprG will lead to reduced expression of virulence factors. In addition, stimulate host cells to mature their phagosomes and/or induce autophagy, both to promote (auto-)phagosomal degradation further impairs intracellular survival of mycobacteria. Drugs can either target mtb (blue/grey drug symbol) or the host (blue/red drug symbol). ‘?’ responsible protein not identified. Created with Bio-Render.com.