Figures & data
Figure 1. Number of HTS assays available for the three triazoles within each endocrine activity hypothesis. The assay endpoints available by estrogen (E), anti-estrogen (AE), androgen (A), anti-androgen (AA), steroidogenesis (S), thyroid receptor (TR), anti-thyroid receptor (ATR) and hepatic catabolism related to thyroid hormone (Liver). Not all triazoles were tested in all assays, leading to minor discrepancies between triazoles for the number of assay endpoints available.
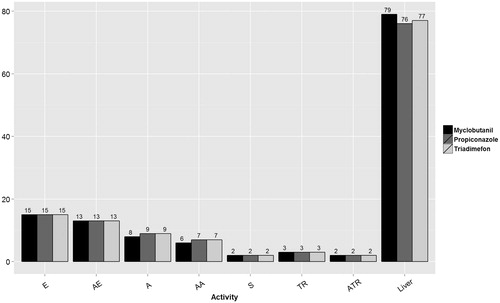
Figure 2. Triadimefon: Parallel comparison of predicted bioactivity and exposure and US EPA chronic exposure estimates and in vivo bioactivity. Annotated to show Bins 1–2. See text, , and Supplemental File 2 for additional details on AC50 by assay and bin. Dotted line = cytotoxicity caution flag (1.68 μM or 5.13 mkd). LPEAD, lowest potentially endocrine active dose (= 1800 ppm or 100 mkd for triadimefon).
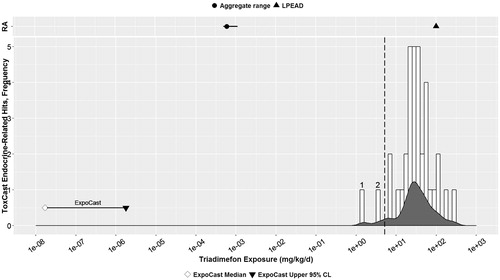
Figure 3. Propiconazole: Parallel comparison of predicted bioactivity and exposure and EPA chronic exposure estimates and in vivo bioactivity. Annotated to show Bins 1–8. See text, , and Supplemental File 2 for additional details on AC50 by assay and bin. Dotted line = cytotoxicity caution flag (4.6 μM or 4.15 mkd). LPEAD, lowest potentially endocrine active dose (= 2500 ppm or 200.4 mkd for propiconazole).
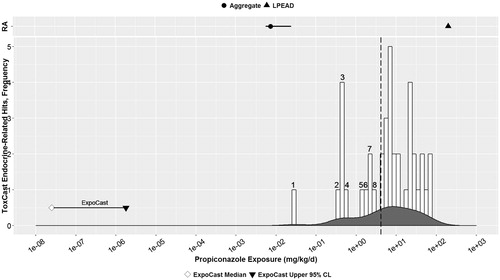
Figure 4. Myclobutanil: parallel comparison of predicted bioactivity and exposure and EPA chronic exposure estimates and in vivo bioactivity. Annotated to show Bins 1–4. See text, , and Supplemental File 2 for additional details on AC50 by assay and bin. Dotted line = cytotoxicity caution flag (4.69 μM or 5.13 mkd). LPEAD, lowest potentially endocrine active dose (= 200 ppm or 9.84–12.86 mkd for myclobutanil). For graphing purposes, the midpoint of the interval between 9.84 and 12.86, or 11.35 mkd, was used as the LPEAD.
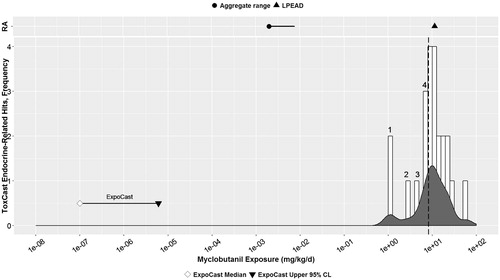
Table 1. Steady state systemic concentration values used to calculate oral equivalent doses.
Table 2. Predicted human oral exposure range for three triazoles.
Table 3. Chronic aggregate exposure estimates from EPA risk assessments.
Table 4. EDSP Tier 1 assays plus published OSRI for the three triazoles.
Table 5. Guideline toxicity studies.
Table 6. Triadimefon: Endocrine-related HTS bioactivity data.
Table 7. Propiconazole: Endocrine-related HTS bioactivity data.
Table 8. Myclobutanil: endocrine-related HTS bioactivity data.
Table 9. Triadimefon: mammalian EDSP Tier 1 assay results plus published OSRI.
Table 10. Propiconazole: mammalian EDSP Tier 1 assay results plus published OSRI.
Table 11. Myclobutanil: mammalian EDSP Tier 1 assay results plus published OSRI.
Table 12. Triadimefon: Part 158 Guideline Toxicology Studies.
Table 13. Propiconazole: Part 158 Guideline Toxicology Studies.
Table 14. Myclobutanil: Part 158 Guideline Toxicology Studies.
Table 15. Triadimefon: Bioactivity concordance in mammalian toxicology assays across three lines of evidence.
Table 16. Propiconazole: Bioactivity concordance in mammalian toxicology assays across three lines of evidence.
Table 17. Myclobutanil: Bioactivity concordance across three lines of evidence.
Table 18. AUC model and IBER values for the three triazoles.
Table 19. AC50 values in HTS aromatase inhibition assays for the three triazoles
Table 20. AC50 values in HTS assays for UGT1A1 and SULT2A1 induction.
Figure 5. Proposed roadmap for EDSP evaluation of pesticide active ingredients (PAIs). For PAIs, the available high-throughput (HT) hazard and exposure information would be considered first, followed by evaluation of the available guideline toxicology study information before making any priority decisions. Priority decisions could include decisions to collect additional hazard or exposure information, or to make a risk assessment (RA) decision based on adequate available data or suitable margins of separation between predicted exposure and bioactivity.