Figures & data
Figure 1. Key hurdles of antitumor immunity in B cell malignancies and how CAR technology may solve them (A), and schematic representation of a CAR (B).
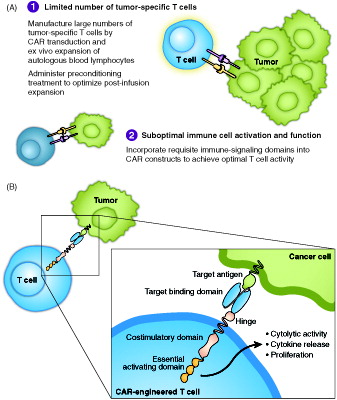
Figure 2. Treatment schema and outcomes for refractory DLBCL. ASCT: autologous stem cell transplant; DLBCL: diffuse large B cell lymphoma; Rtx: rituximab.
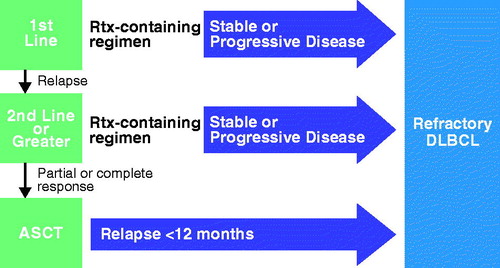
Figure 3. CAR gene (A) and manufacturing process (B) for axicabtagene ciloleucel. (A) Graphical representation of the CAR gene introduced into human T cells using a retroviral vector to make axicabtagene ciloleucel. The gene encodes a protein of ∼54 kDa. SP indicates the position of the signal peptide. CD28-TM-CD28 indicates the position of the extracellular, transmembrane (TM), and intracellular regions of CD28, respectively. bp: base pairs; LC: light chain; HC: heavy chain. (b) Schematic overview of the vein to vein axicabtagene ciloleucel production process. The process begins with collection of blood cells at the clinical center. Patient material is then transported to the central manufacturing site where axicabtagene ciloleucel is produced. T cells in the incoming leukapheresis material are enriched on a closed-system density gradient. T cells are then activated with anti-CD3 antibody in the presence of IL-2 for 48 h, after which time they become receptive to transduction with a gamma-retroviral vector that encodes the anti-CD19 CAR gene. After transduction, cells are expanded until a target dose is achieved (2 × 106 CAR-positive cells per kg body weight). After product release, the final product is returned to the clinical center. Door-to-door turn around time is approximately 2 weeks.
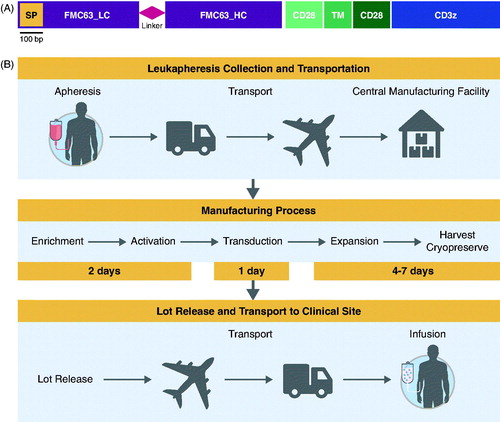
Table 1. Axicabtagene ciloleucel in single-center and national clinical trials.
Figure 4. Kinetics of key cytokines and chemokines during CAR T cell therapy. CRP: C-reactive protein; d: day; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN: interferon; IL: interleukin; MIP: macrophage inflammatory protein.
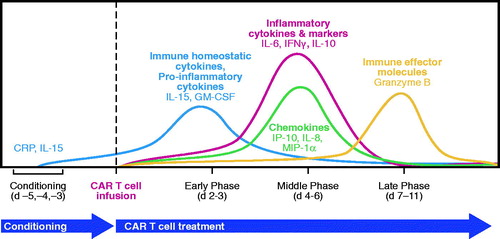
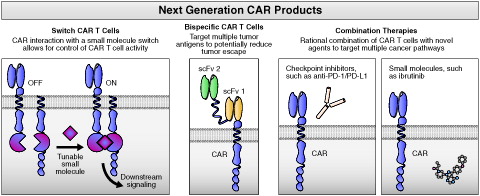
Figure 5. Major future directions in the development of next-generation CAR products. CAR: chimeric antigen receptor; PD-1: programed death receptor; PD-L1: programed death receptor ligand; scFv: single chain variable fragment.