Figures & data
Table 1. IPSS-R classification for MDS [Citation4].
Figure 1. Study design. aMDS chemotherapy includes the following: azacitidine, decitabine, lenalidomide, ATG, cyclosporine, cytarabine, idarubicin, daunorubicin, fludarabine, clofarabine and topotecan, or alloSCT. bMDS-specific supportive care includes the following: erythrocyte/platelet transfusions; thrombopoietic-, erythropoietic-, and granulocyte-stimulating agents, and hydroxyurea. AML: acute myeloid leukemia; alloSCT: allogeneic stem cell transplant; ATG: anti-thymocyte globulin; HR-MDS: higher-risk myelodysplastic syndrome; ICD-9/ICD-10: International Classification of Diseases 9th/10th Revision; IDN: integrated delivery network; MDS: myelodysplastic syndrome; SCT: stem cell transplant.
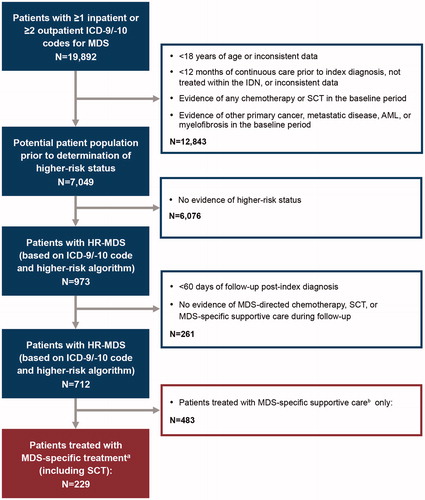
Table 2. Baseline patient characteristics and treatment characteristics.
Figure 2. Prescribing patterns for patients receiving second-line therapy. aInduction-type treatment: an antimetabolite (i.e. cytarabine, fludarabine) ± an anthracycline (i.e. idarubicin or daunorubicin). bCytotoxic chemotherapy: any non-HMA chemotherapy (i.e. fludarabine ± another agent, clofarabine, cytarabine ± daunorubicin). cOf the lenalidomide patients, n = 4 were lenalidomide alone and n = 2 were in combination with azacitidine. HMA: hypomethylating agent.
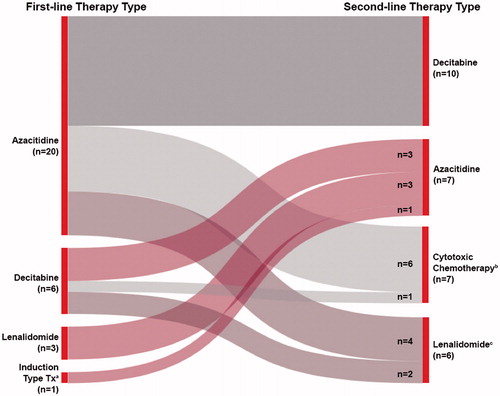
Figure 3. Progression-free survival (A) and overall survival (B) in patients with and without a TFI receiving first-line therapy (p < .0001 for both). TFI: transfusion-free interval.
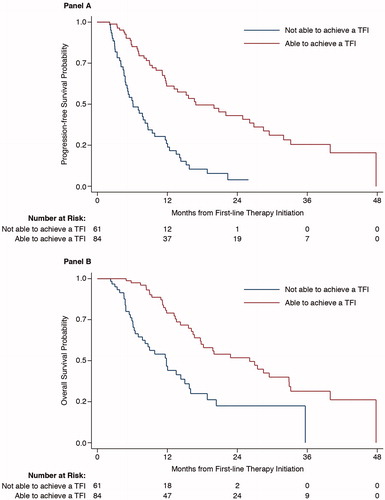
Table 3. Multivariate analysis of 2-year PFSTable Footnotea,Table Footnoteb and OSTable Footnotea,Table Footnoteb among HR-MDS patients with and without a TFI.
