Figures & data
Table 1. Baseline patient characteristics.
Figure 1. TFR in all enrolled patients at 2 years (N = 84). CI: confidence interval; NE: not estimable; TFR: treatment-free remission.
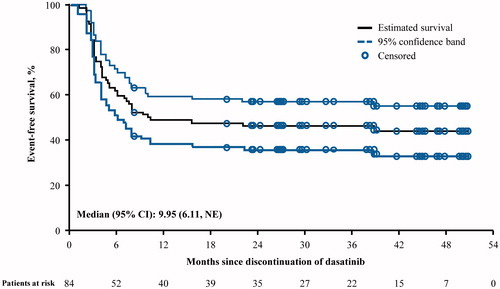
Figure 2. Loss and recovery of MMR and MR4.5 at 2 years. aA 52-year-old male with low-risk CML treated with first-line dasatinib 100 mg once daily for 40 months discontinued the study after 33 months in MR4.5. This patient maintained BCR-ABL1 transcript levels between 0.0017 and 0.01 for 3 years and had an increase to 0.1% in month 39, which was confirmed on a second occasion in month 42 (0.11%). bOne patient lost MMR and restarted treatment. This patient discontinued the study after only one follow-up molecular assessment and therefore was not considered evaluable for molecular response. CML: chronic myeloid leukemia; MMR: major molecular response; MR4.5: BCR-ABL1 ≤ 0.0032% on the International Scale.
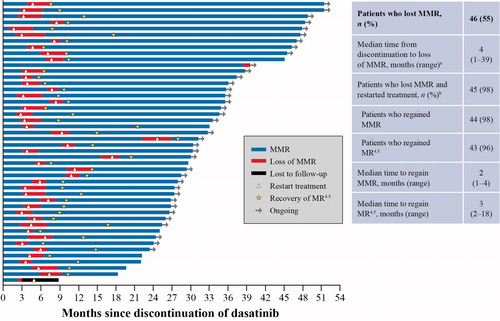
Figure 3. Multivariate analysis of TFR and covariates at 2 years. CI: confidence interval; MR4.5: BCR-ABL1 ≤ 0.0032% on the International Scale; TFR: treatment-free remission; TKI: tyrosine kinase inhibitor.
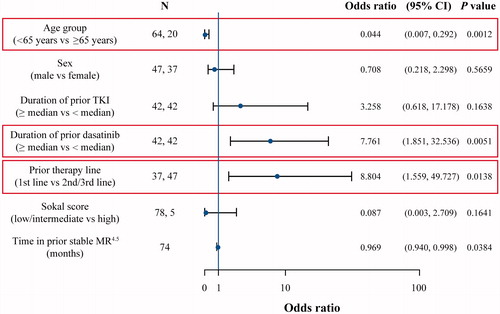
Table 2. All causality adverse events in ≥4% of enrolled patients and withdrawal events in all enrolled patients.
Supplemental Material
Download JPEG Image (866.4 KB)Supplemental Material
Download JPEG Image (1.1 MB)GLAL-2019-0709-File009.docx
Download MS Word (43 KB)GLAL-2019-0709-File008.docx
Download MS Word (43.8 KB)GLAL-2019-0709-File007.docx
Download MS Word (347.3 KB)Data availability statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
