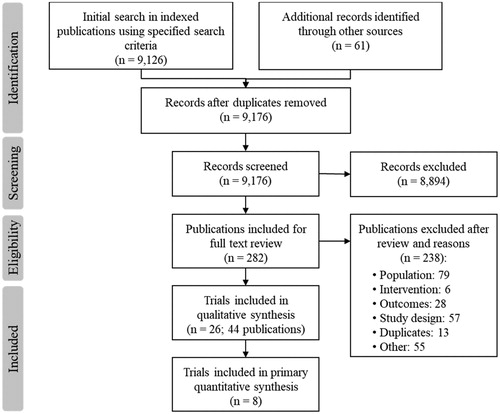
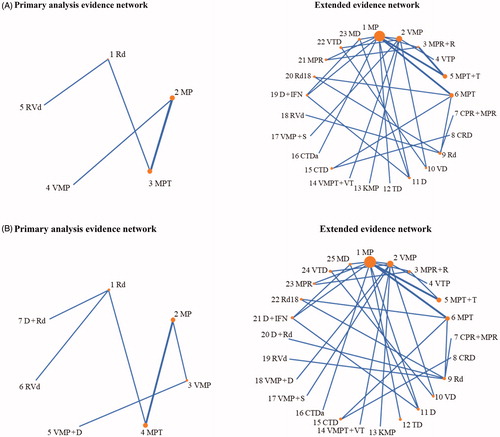
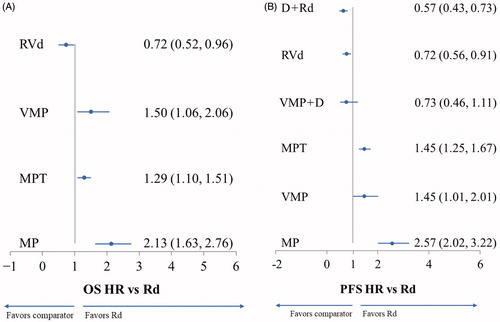
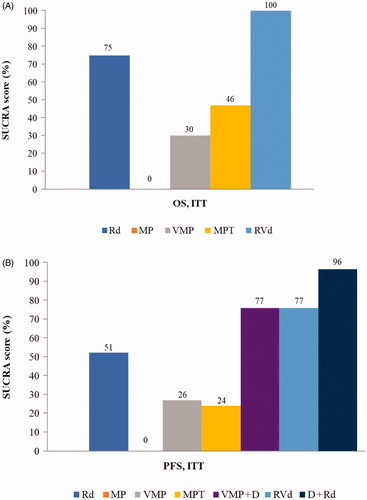
Figures & data
Table 1. Extracted efficacy data from RCTs in the primary and extended analysis networks.
Table 2. Baseline age and ISS stage from RCTs in the primary analysis network.