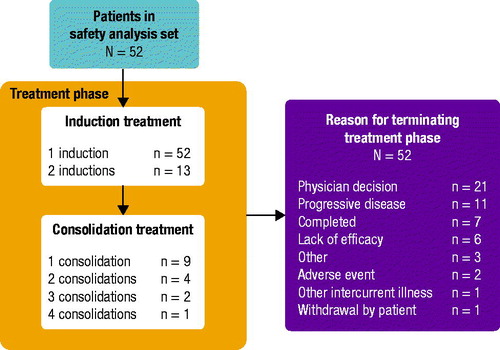
Figures & data
Table 1. Baseline demographic and clinical characteristics.
Figure 2. Most frequently reported TEAEs by maximum severity grade. All TEAEs reported by ≥5% of patients are included and shown by maximum Common Terminology Criteria for Adverse Events severity grade. TEAE: treatment-emergent adverse event.
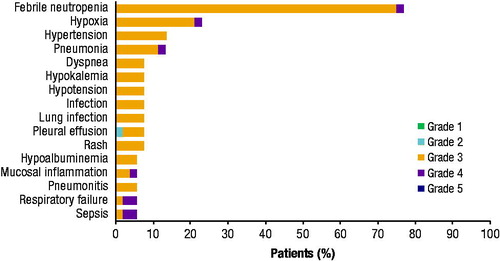
Table 2. Summary of TEAEs.
Table 3. Median time to neutrophil and platelet recovery in patients who achieved CR or CRia.
Table 4. Best induction response rates.
Data availability
All relevant data are provided within the manuscript and supporting files.