Figures & data
Figure 1. Agents approved for treatment of newly diagnosed patients with AML ineligible for intensive chemotherapy. AML: acute myeloid leukemia; IC: intensive chemotherapy; IDH: isocitrate dehydrogenase; LDAC: low-dose cytarabine.
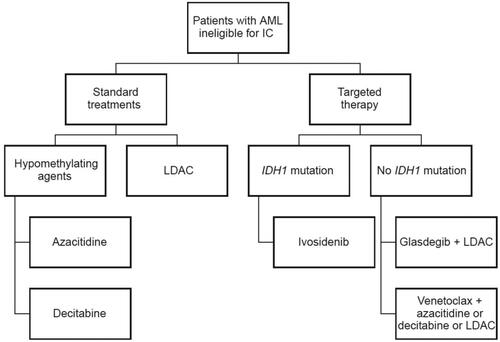
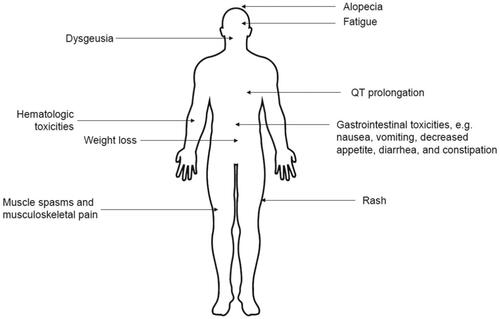
Table 1. Summary of agents approved for treatment of patients with AML ineligible for intensive chemotherapy.
Table 2. Summary of eligibility criteria in key clinical trials in patients with AML who are ineligible for intensive treatment.
Table 3. Summary of baseline characteristics in key clinical trials in patients with AML who are ineligible for intensive treatment.
Table 4. Summary of results from patients ineligible for IC in BRIGHT MDS&AML 1003 (including unpublished data) [Citation40,Citation50–52].
Table 5. Summary of considerations for glasdegib use.
Table 6. Summary of the management of the most common AEs associated with glasdegib [Citation35,Citation81–84].