Figures & data
Figure 1. Overall response rate (ORR) and progression-free survival (PFS) analyses. (A) Unadjusted and adjusted ORR and PFS. The ORR and PFS and associated 95% confidence intervals (CIs) are shown for twice-weekly (BIW) KdD56 and once-weekly (QW) KdD70. Also shown are the odds ratios (ORs; for ORR) and hazard ratios (HRs; for PFS) and associated 95% CIs. Sensitivity analyses for ORR (B) and PFS (C), with corresponding OR for ORR and HR for PFS, and associated 95% CIs are shown for BIW KdD56 and QW KdD70. Progression-free survival (PFS) for unadjusted primary analysis (D) and IPTW-IRC analysis (E).
BIW: twice-weekly; CI: confidence interval; HR: hazard ratio; IRC: Independent Review Committee; IPTW: inverse probability of treatment weighting; KdD: carfilzomib, dexamethasone, and daratumumab; NE: not estimable; QW: once weekly.
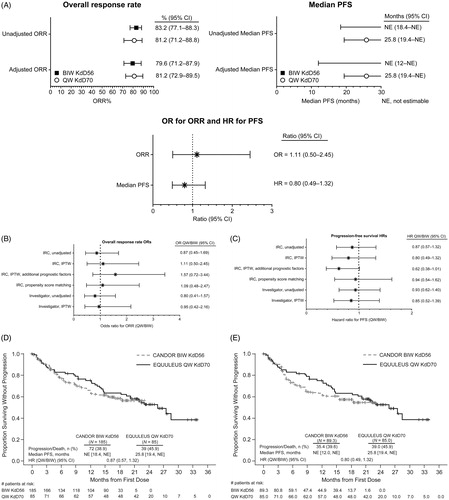
Table 1. Unadjusted baseline demographics and disease characteristics.
Table 2. Baseline demographics and disease characteristics of adjusted BIW KdD56 and QW KdD70 groups after using propensity score.
Table 3. Unadjusted and adjusted ORR and PFS.
Table 4. Efficacy results for selected subgroups.
Table 5. Safety.
