Figures & data
Figure 1. Percentage of patients and progression-free survival by delay. (A) Delay from diagnosis-to-randomization. (B) Delay from diagnosis-to-initiation of screening. (C) Delay from initiation of screening to randomization.
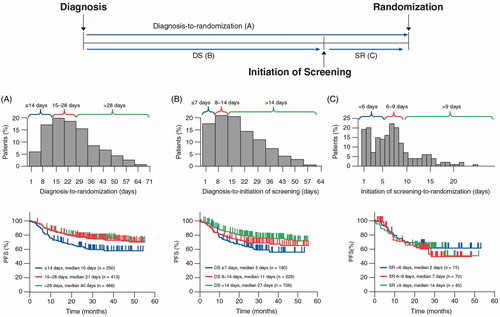
Table 1. Patient and disease characteristics by time from diagnosis-to-initiation of screening (safety population).
Data availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
