Figures & data
Figure 1. GIBB study design for previously untreated patients with chronic lymphocytic leukemia receiving obinutuzumab/bendamustine (NCT02320487). BM: bone marrow; CLL: chronic lymphocytic leukemia; d: day; iwCLL: International Working Group guidelines for CLL; mo: month; MRD: minimal residual disease; PB: peripheral blood; y: year.
Table 1. Baseline patient demographics and disease characteristics in patients receiving obinutuzumab/bendamustine in the GIBB study (intent-to-treat population).
Figure 2. Time-to-event results for patients receiving obinutuzumab/bendamustine in the GIBB study for (A) duration of response, (B) progression-free survival, and (C) overall survival (modified intent-to-treat population). Data cutoff: 3 June 2019.
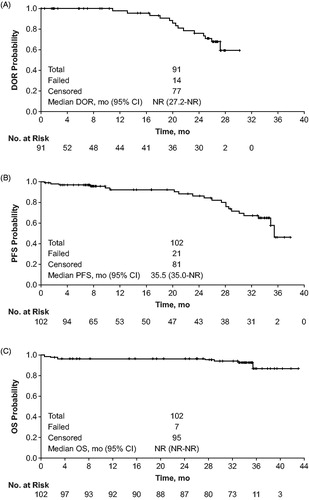
Table 2. Response at treatment completion in patients receiving obinutuzumab/bendamustine in the GIBB study (modified intent-to-treat population).
Figure 3. Time to and duration of MRD negativity (<10−4) status in patients receiving obinutuzumab/bendamustine in the GIBB study in peripheral blood: (A) time to MRD negativity (<10−4) following initial positive MRD assessment and (B) duration of MRD-negativity defined as the time from first occurrence of MRD-negative status to subsequent MRD-positive status. Data cutoff: 3 June 2019.
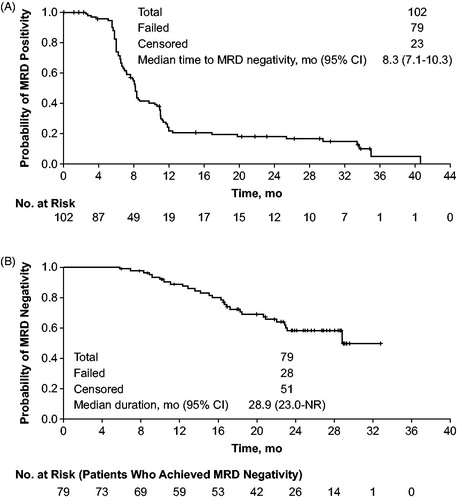
Table 3. Treatment-emergent adverse events (TEAEs; ≥ 5% any grade) in patients receiving obinutuzumab/bendamustine in the GIBB study (safety population; N = 102).
Data availability statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available here: (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
