Figures & data
Table 1. Baseline demographics in the intent-to-treat population.
Table 2. Secondary efficacy variables in the intent-to-treat population.
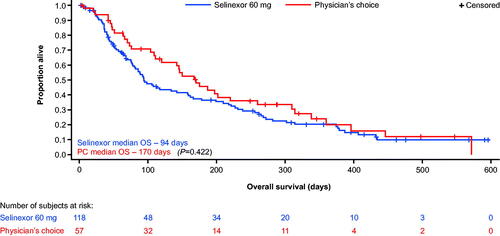
Figure 2. Overall survival in patients with and without CR plus CRi/CRp in the intent-to-treat population. mCRR = CR + CRi + CRp. CR: complete remission; CRi: complete remission with incomplete recovery; CRp: complete remission with incomplete platelet recovery; mCRR: modified complete remission rate; PC: treatment of physician’s choice.
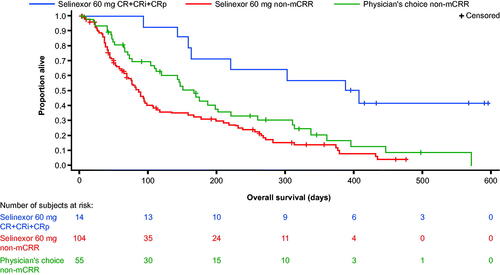
Table 3. Summary of TEAEs occurring in the safety population.
Table 4. Most common TEAEs occurring in ≥10% of patients in any treatment group in the safety population.
ilal_a_1950706_sm2478.docx
Download MS Word (523.6 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary material.