Figures & data
Figure 1. Cumulative best response rate over time by first-line (A), 1-2 prior lines (B), and ≥3 prior lines of therapy (C). Response assessed by investigators by study time points. CR: complete response; CRi: complete remission with incomplete bone marrow recovery; PR: partial response; PRL: PR with lymphocytosis; nPR nodular PR.
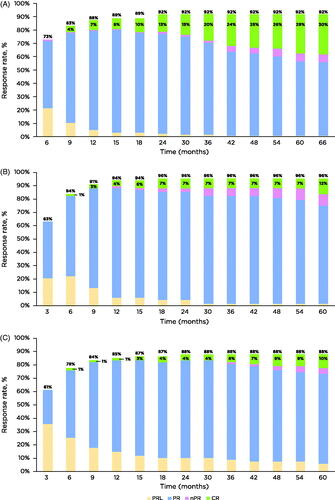
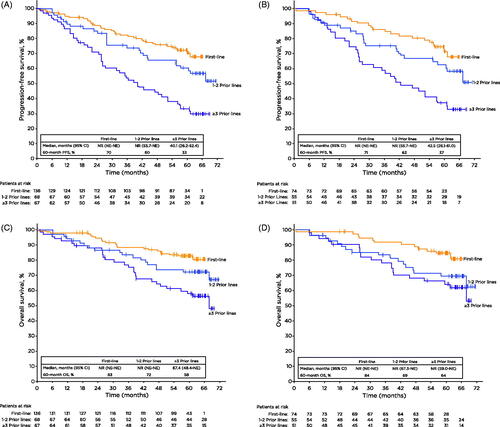
Figure 2. Progression-free survival (A-B) and overall survival (C-D) by prior lines of therapy for all patients and high-risk disease features. Tick marks on the curves indicate patients with censored data. CI: confidence interval; NE: not estimable; NR: not reached; OS: overall survival; PFS: progression-free survival.
GLAL-2021-0357-File004.docx
Download MS Word (273.8 KB)Data availability statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
