Figures & data
Table 1. Baseline patient characteristics.
Table 2. Hematologic malignancy treatments by cancer subtype and by IgRT group during the baseline period.
Figure 1. Percentage of patients with bacterial infections during the baseline period, in IgRT or non-IgRT users. *All p<.0001 for IgRT versus non-IgRT users; except for severe BI (p=.0011). BI: bacterial infections; IgRT: immunoglobulin replacement therapy.
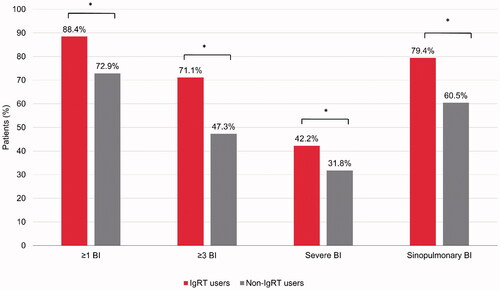
Figure 2. Mean number of bacterial infections per patient, in the 12-month baseline and follow-up periods (observed and predicted) for the overall cohort and cancer-specific subtypes. Follow-up period predicted values were estimated using a GLM approach with a Poisson distribution and log-link transformation. BI: bacterial infections; CLL: chronic lymphocytic leukemia; GLM: generalized linear regression model; IgRT: immunoglobulin replacement therapy; MM: multiple myeloma; NHL: non-Hodgkin lymphoma.
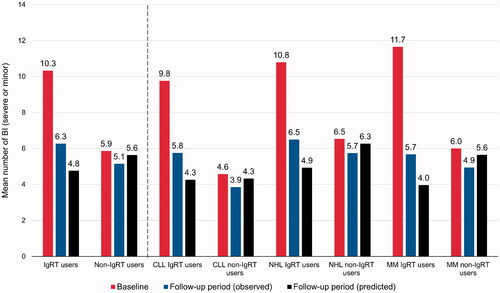
Figure 3. Percentage of patients with ≥1 severe bacterial infection during the baseline and follow-up periods, in IgRT or non-IgRT users. BI: bacterial infection; CLL: chronic lymphocytic leukemia; IgRT: immunoglobulin replacement therapy; MM: multiple myeloma; NHL: non-Hodgkin lymphoma.
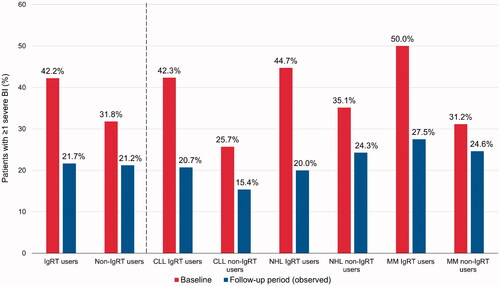
Figure 4. Mean number of severe bacterial infections per patient, in the 12-month baseline and follow-up periods (observed and predicted) for the overall cohort and cancer-specific subtypes. Follow-up period predicted values were estimated using a GLM approach with a Poisson distribution and log-link transformation. The impact of IgRT on severe BI was statistically significant during the follow-up period in the overall cohort and for the NHL and CLL subtypes, but not for the MM subtype, based on evidence from the corresponding GLM models. BI: bacterial infections; CLL: chronic lymphocytic leukemia; GLM: generalized linear regression model; IgRT: immunoglobulin replacement therapy; MM: multiple myeloma; NHL: non-Hodgkin lymphoma.
Supplemental Material
Download MS Word (37.1 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author, RM, upon reasonable request.
