Figures & data
Figure 2. Patient flow diagram. AML: acute myeloid leukemia; BSC: best supportive care; CML: chronic myeloid leukemia; ECOG: Eastern Cooperative Oncology Group; HIC: high intensity chemotherapy; LIC: low intensity chemotherapy; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; TT: targeted therapy. (a) The fifth digit of the diagnosis code for AML can be 0 (not having achieved remission), 1 (in remission), or 2 (in relapse). (b) If the first claim is from Part A outpatient or Part B, presence of a second AML claim from any source carrying the diagnosis code for not having achieved remission (.X0) within 30 days of the initial diagnosis is required. (c) Presence of a confounding disease was defined by ≥1 Part A inpatient, skilled nursing facility, home health agency, or hospice claim with specific diagnosis codes for the disease, or ≥2 Part A outpatient or Part B claims with specific diagnosis codes for the disease on different days up to 30 days. This definition was applied for each individual disease during the baseline period and the follow-up period. (d) Other confounding diseases include: neoplasm of uncertain or unspecified behavior, acute lymphoid leukemia, lymphoid leukemia, monocytic leukemia, other leukemia, eosinophilia, gastrointestinal stromal tumor, malignant mast cell tumors. (e) Criteria for ‘ineligible for standard induction chemotherapy’ include: age >75 years at AML diagnosis, cardiac disease or prior anthracycline, secondary AML.
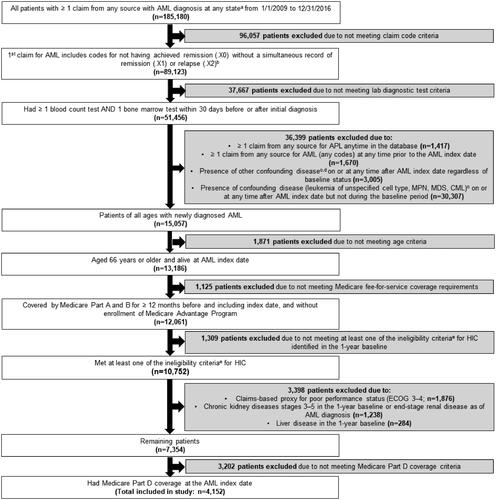
Table 1. Patient characteristics of elderly patients newly diagnosed with AML between 1 January 2009 and 31 December 2016, overall and by treatment status.
Table 2. Chemotherapy treatment patterns for patients (n = 1,792) receiving chemotherapy within 60 days of AML index date.
Table 3. Unadjusted cumulative incidence of all-cause death and median OS from chemotherapy or BSC initiation.