Figures & data
Table 1. Clinical characteristics at inclusion.
Table 2. Adverse events.
Figure 1. Peak plasma levels of THC and CBD, number of actuations and adverse events. (A) Peak plasma levels of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), each dot representing a patient. (B) Peak plasma levels of THC and the number of actuations of THC/CBD and the worst adverse event per patient (gray dot, no adverse event; dark gray triangle, adverse event grade 1; black diamond, adverse event grade 2).
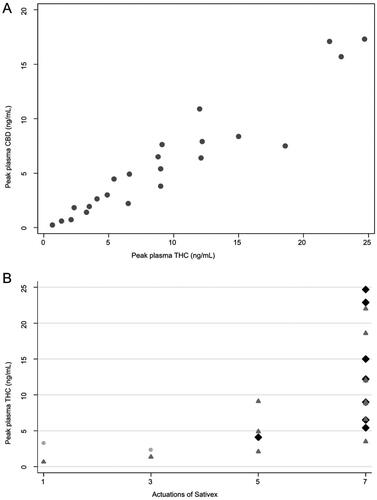
Figure 2. Changes in median levels of blood leukocyte subsets and serum cortisol levels during the days without and with THC/CBD. For simplicity, only median values are shown here, detailed data are represented in . (A) Leukemic B cells. (B) Normal B cells. (C) CD3+ T cells. (D) CD4/CD8 ratio. (E) CD56+ NK cells. (F) Platelets. (G) Neutrophils. (H) Serum cortisol. Dashed black lines are from the day without treatment (n = 13) and solid gray lines are from the day with treatment (n = 23). All results are presented in relation to sampling at baseline (9 am) for each day. All cell-subset analyses were calculated from absolute blood counts, except CD4/CD8 (ratio). One circumflex (^) indicates significant changes at the day without treatment, with respect to baseline with p < .05. One asterisk (*) and two asterisks (**) indicate significant changes at the day with treatment, with respect to baseline with p < .05 and p < .005, respectively.
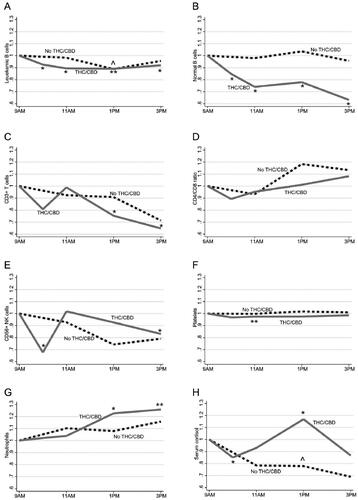
Table 3. Changes with and without THC/CBD.
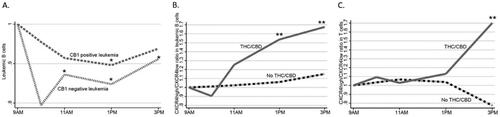
Figure 3. Reduction of CB1-expressing and non-expressing malignant B cells and CXCR4 surface expression. For simplicity, only median values are shown here, detailed data are represented in . (A) Reduction of leukemic B cells after THC/CBD in CB1 positive (dashed gray line; n = 17) and CB1 negative (dotted gray line; n = 6) cases. Changes in CXCR4+/CXCR4- ratio in (B) leukemic B cells and (C) T cells.