Figures & data
Figure 1. Exposure and best response in all evaluable patients (A) and transfusion requirements (B). mCR: bone marrow complete response; PD: progressive disease.
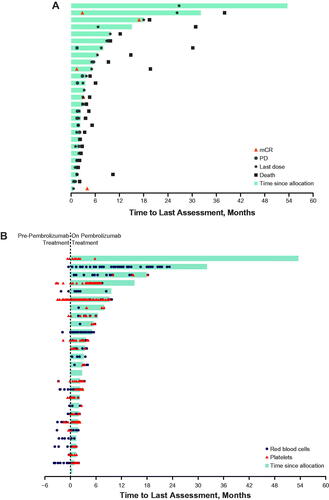
Table 1. Baseline characteristics of all patients as treated.
Table 2. Treatment-related adverse events in all patients as treated.
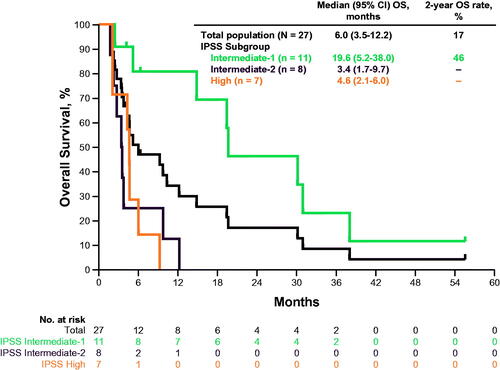
Figure 2. Percentage by best response in all evaluable patients. (A) Bone marrow blasts. (B) Blasts/leukocytes. mCR, bone marrow complete response; PD: progressive disease; SD: stable disease.
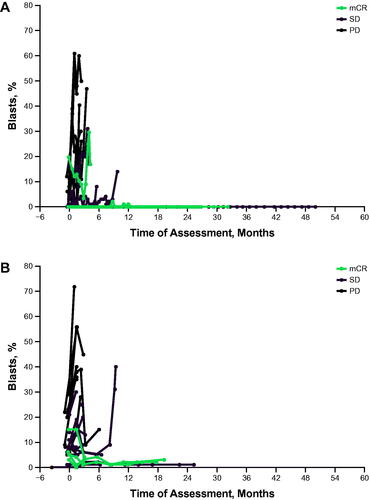
Table 3. Response and hematologic improvement in all evaluable patients per IWG 2006 criteriaa.
Data availability statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.