Figures & data
Table 1. Baseline characteristics and demographics of patients treated with ibrutinib in clinical trials.
Figure 1. Percentage of patients with the first onset of low-grade (grade 1/2) arthralgia, myalgia, and musculoskeletal pain over time (N = 1178).
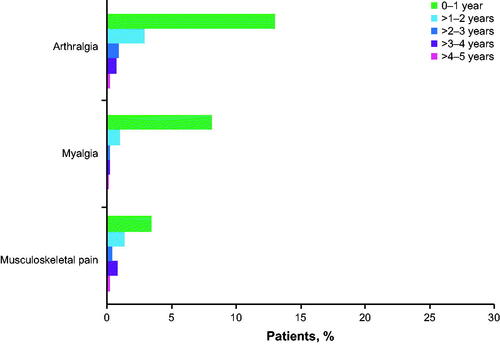
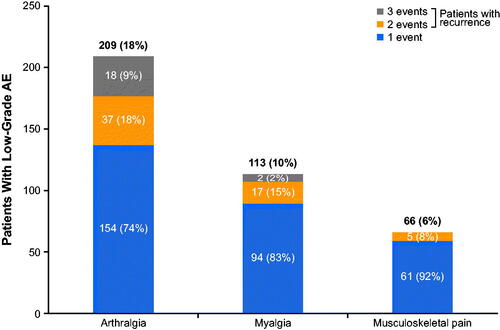
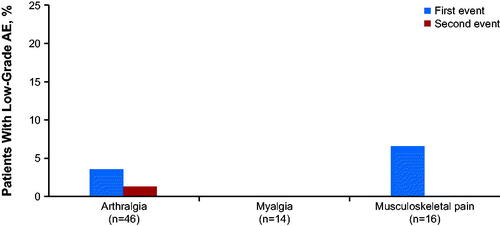
Table 2. Summary of arthralgia, myalgia, and musculoskeletal pain and concomitant medication use for patients treated with ibrutinib (RESONATE-2/iLLUMINATE dataset).
GLAL-2021-0948-File002.docx
Download MS Word (2.6 MB)Data availability statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu