Figures & data
Table 1. Patients with adverse events from part 1 (patients with R/R B-cell NHL receiving acalabrutinib 100 mg BID and 25, 50, or 100 mg ACP-319 BID) and part 2 (patients with GCB DLBCL or non-GCB DLBCL receiving acalabrutinib 100 mg BID and 50 mg ACP-319 BID).
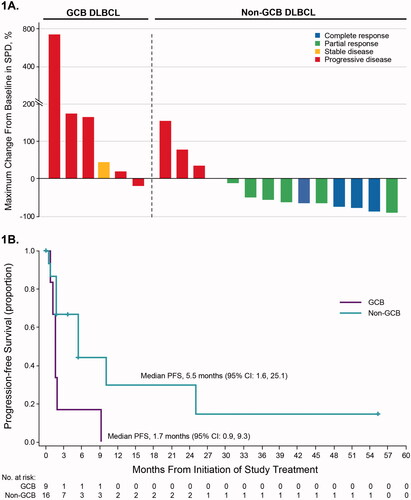
Figure 1. Efficacy outcomes in patients with non-GCB or GCB subtype DLBCL (part 2). (A) Responses and percentage change in tumor burden in individual patients (n = 20). Data are not shown for 3 patients with GCB subtype DLBCL and 2 patients with non-GCB DLBCL, who were not on study long enough to complete response assessment and were thus not evaluable. These patients were treated for ≤42 days. (B) Progression-free survival. Abbreviations: CR: complete response; DLBCL: diffuse large B-cell lymphoma; GCB: germinal center B-cell; PD: progressive disease; PFS: progression-free survival; PR: partial response; SD: stable disease; SPD: sum of product diameters.
GLAL-2021-1043-File003.pdf
Download PDF (150.1 KB)Data-sharing statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
