Figures & data
Figure 1. Momelotinib-treated patients experienced increased hemoglobin levels and reduced transfusion requirements over those seen in ruxolitinib-treated patients in SIMPLIFY-1. (A) Mean hemoglobin concentrations over time for patients receiving momelotinib or ruxolitinib. In the 24-week double-blind period a statistically significant increase in mean hemoglobin concentration was observed in patients receiving momelotinib (p < 0.001), in contrast to a statistically significant decrease in patients receiving ruxolitinib (p < 0.001). Patients crossing over from ruxolitinib to momelotinib at the end of randomized treatment experienced a rapid and sustained increase in hemoglobin, with levels approaching those in patients originally randomized to momelotinib. (B–D) KM survival function estimates of (B) time-to-first, (C) time-to-third, and (D) time-to-fifth RBC transfusions for patients receiving momelotinib versus ruxolitinib, with 95% CIs shaded. (B) 73% of patients on momelotinib compared to 46% on ruxolitinib did not receive a transfusion unit by week 24 (log-rank p < 0.0001). (C) Odds of receiving <3 transfusion units were 3.6 times higher on momelotinib (81%) compared to ruxolitinib (54%, log-rank p < 0.0001). (D) Odds of receiving <5 transfusion units were 3.0 times higher on momelotinib (83%) compared to ruxolitinib (62%, log-rank p < 0.0001). (E) Cumulative transfusion burden for patients receiving momelotinib versus ruxolitinib, with 95% CIs shaded. The HR for a RBC unit transfused for patients receiving momelotinib was approximately one-half that for patients receiving ruxolitinib (HR 0.522; p < 0.0001), suggesting that at any time point, an average patient on ruxolitinib would receive twice as many RBC transfusions compared with an average patient on momelotinib. (F) KM survival function estimate of the durability of transfusion-independence for patients randomized to momelotinib. Time from first transfusion-independence rolling 12-week response (week 0 to week 24) to first failure (any time; event = any transfusion OR hemoglobin level <8 g/dL) is shown. The median time-to-loss of transfusion-independence was not reached for randomized momelotinib-treated patients, with a follow-up period exceeding 3 years. CI: confidence interval; HR: hazard ratio; KM: Kaplan-Meier; RBC: red blood cell.
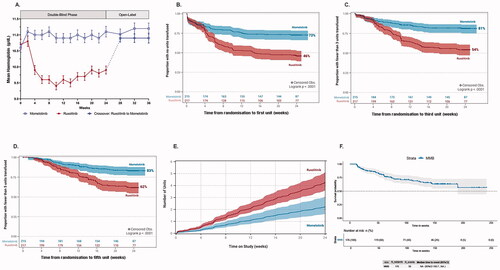
Table 1. Probability of zero transfusions was greater for patients receiving momelotinib versus ruxolitinib in SIMPLIFY-1.
Data availability statement
Sierra Oncology, Inc., supports the principles of clinical trial data transparency, including registration and disclosure of clinical trial results in external registries and publication of results in peer-reviewed journals. Clinical trial results for SIMPLIFY-1 (NCT01969838) have been previously published (Mesa RA, et al. J Clin Oncol. 2017;35:3844-3850) and will be posted to ClinicalTrials.gov and other public registries in accordance with the requirements of each jurisdiction in which the study was conducted. Inquiries regarding availability of data and clinical trial documentation should be directed to: [email protected].
