Figures & data
Table 1. Patient demographics at the time of enrollment.
Figure 1. Patients with MF: mean (SD) MPN-SAF TSS patient-reported outcome scores for (A) total evaluable population, (B) total evaluable population, by sex, and (C) total evaluable population, by risk category; (D) MPN-SAF symptom severity at enrollment. *p < 0.05. INT: intermediate; MF: myelofibrosis; MPN-SAF TSS: Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score; SD: standard deviation.
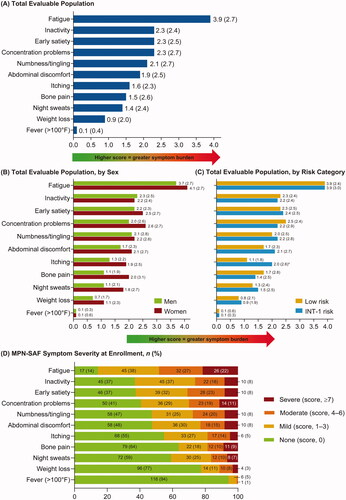
Figure 2. Patients with ET: mean (SD) MPN-SAF TSS patient-reported outcome scores for (A) total evaluable population, (B) total evaluable population, by sex, and (C) total evaluable population, by risk category; (D) MPN-SAF symptom severity, at enrollment. *p < 0.05, **p < 0.01. ET: essential thrombocythemia; MPN-SAF TSS: Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score; SD: standard deviation.
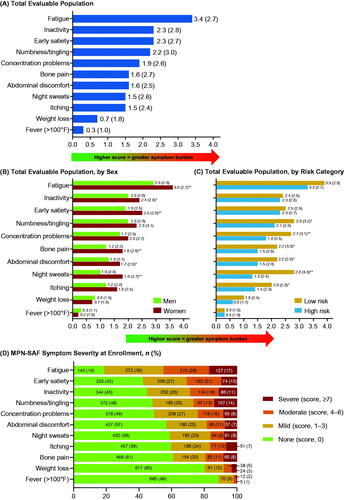
Figure 3. Patients with MF: mean (SD) EORTC QLQ-C30 scores at enrollment for symptom scale scores for (A) total evaluable population, (B) total evaluable population, by sex, and (C) total evaluable population, by risk category; subscale scores for (D) total evaluable population, (E) total evaluable population, by sex and, (F) total evaluable population, by risk category. *p < 0.05. EORTC QLQ-C30: European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; INT: intermediate; MF: myelofibrosis; QoL: quality of life; SD: standard deviation.
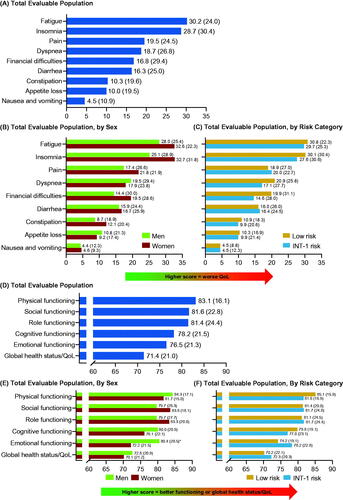
Figure 4. Patients with ET: mean (SD) EORTC QLQ-C30 scores at enrollment for symptom scale scores for (A) total evaluable population, (B) total evaluable population, by sex and, (C) total evaluable population, by risk category; subscale scores for (D) total evaluable population, (E) total evaluable population, by sex and, (F) total evaluable population, by risk category. *p < 0.05. EORTC QLQ-C30: European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; ET: essential thrombocythemia; QoL: quality of life; SD: standard deviation.
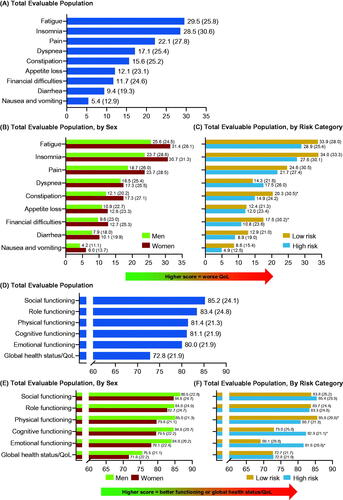
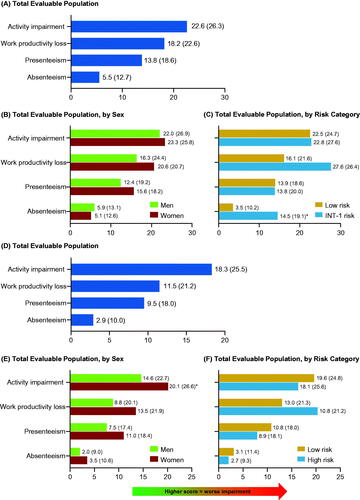
Figure 5. Patients with MF (A–C) or ET (D–F): mean (SD) WPAI-SHP scores for (A, D) total evaluable population, (B, E) total evaluable population, by sex and, (C, F) total evaluable population, by risk category. *p < 0.05. ET: essential thrombocythemia; INT: intermediate; MF: myelofibrosis; SD: standard deviation; WPAI-SHP: Work Productivity and Activity Impairment-Specific Health Problem.
Data availability statement
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g. United States, European Union, Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960
