Figures & data
Table 1. Baseline patient characteristics in the RRHL group.
Table 2. Treatment pathways in the RRHL group.
Figure 1. PFS from initiation of first salvage treatment in the RRHL group. CI: confidence interval; PFS: progression-free survival; RRHL: relapsed/refractory Hodgkin lymphoma. Thirty-six patients with missing dates were excluded from the Kaplan–Meier analysis. East Asia comprises: Republic of Korea, Singapore, Taiwan, China, and Hong Kong; Latin America comprises: Argentina, Colombia, and Mexico; the Middle East and South Africa comprise: Saudi Arabia, Turkey, and South Africa.
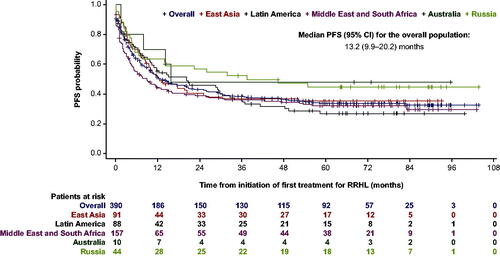
Figure 2. Adjusted hazard ratio for disease progression from initiation of first salvage treatment in patients with RRHL. CI: confidence interval; 1L: first-line; RRHL: relapsed/refractory Hodgkin lymphoma.
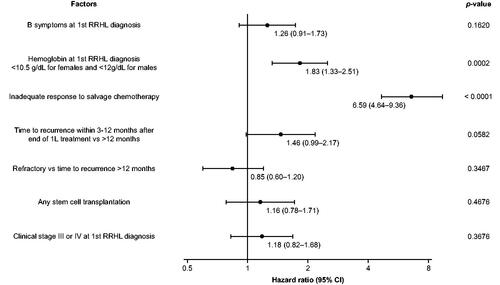
Table 3. Clinical outcomes in the RRHL group.
Table 4. Baseline characteristics, treatment pathways, and clinical outcomes in the frontline cHL group.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article will be made available within 3 months from the initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
