Figures & data
Figure 1. Clinical efficacy outcomes of patients treated with zanubrutinib. (A) Overall response rate assessed by IRC and INV over time. (B) Forest plot of ORR by a predefined subgroup analysis. (C) Kaplan–Meier curves of progression-free survival for the safety population and patients with selected chromosomal abnormalities. For IGHV mutation status, 17 patients were excluded due to the following reasons: three patients with IGHV gene rearrangement undetected, 13 patients with multiclonal IGHV gene rearrangement detected, and one patient with test failed. aTwo-sided Clopper–Pearson 95% CIs. CI: confidence interval; CLL: chronic lymphocytic leukemia; CR: complete response; IGHV: immunoglobulin heavy chain variable region gene; INV: investigator; IRC: independent review committee; NA: not applicable; nPR: nodular partial response; ORR: overall response rate; PFS: progression-free survival; PR: partial response; PR-L: partial response with lymphocytosis; SLL: small lymphocytic lymphoma.
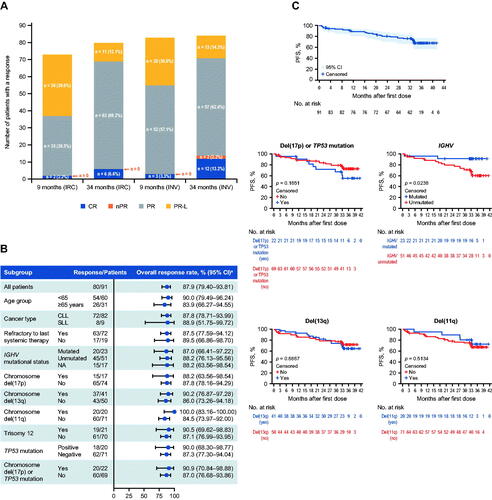
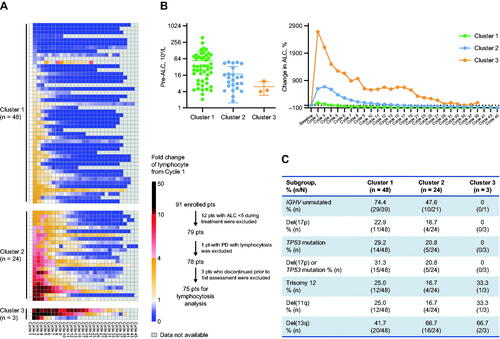
Figure 2. Zanubrutinib-induced lymphocytosis and association with prognostic factors. (A) Identification of three different lymphocytosis patterns by unsupervised cluster analysis using ALC fold change from baseline. (B) Baseline ALC comparison among three lymphocytosis patterns plotted in mean ± SD (left); and percentage change in ALC over different treatment time periods shown in mean value (right). (C) Comparison of baseline prognostic factors among three lymphocytosis patterns. ALC: absolute lymphocyte count; CLL: chronic lymphocytic leukemia; IGHV: immunoglobulin heavy chain variable region gene; PD: progressive disease; pts: patients; SD: standard deviation; SLL: small lymphocytic lymphoma.
GLAL-2022-0640-File004.docx
Download MS Word (120.8 KB)Data availability statement
On request, and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual de-identified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to [email protected]
