Figures & data
Table 1. Baseline characteristics of patients with multiple myeloma.
Table 2. Baseline characteristics of patients treated with daratumumab-containing regimens.
Table 3. Clinical characteristics of patients with EBV and/or CMV infections during daratumumab-containing regimens.
Table 4. Characteristics, treatments, and outcomes of the EBV/CMV infections during daratumumab treatment.
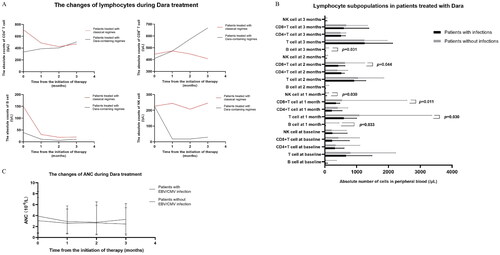
Figure 1. Longitudinal monitoring of lymphocytes and neutrophils in MM patients treated with classical regimens or Dara-containing regimens. A) B cell depletion, NK cell depletion and T cell expansion (particularly CD8 + T cell) were observed in MM patients treated with Dara-containing regimens, which were not observed in MM patients treated with classical regimens. B) Comparison of lymphocyte subpopulations at different time points in the course of daratumumab treatment between MM patients with infections and MM patients without infections. C) The levels of ANC in MM patients in the two groups were comparable in the process of Dara treatments.
Data availability statement
The data of this study are available from the corresponding author upon reasonable request.
