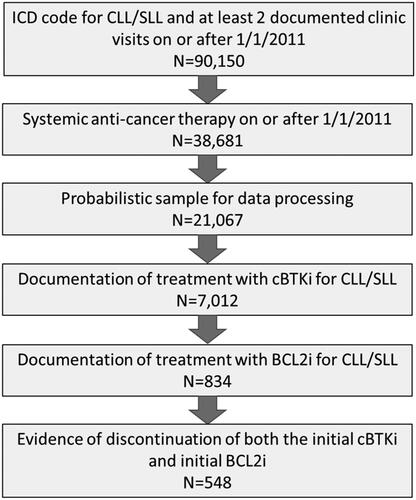
Figures & data
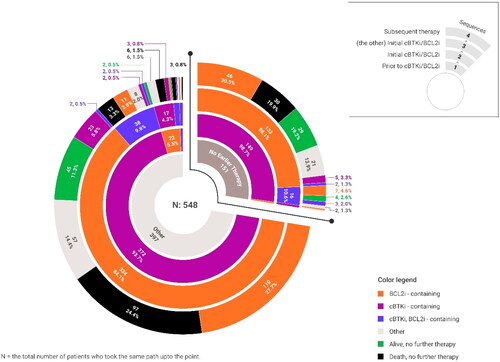
Table 2. Sequence and timing of cBTKi and BCL2i therapy.
Table 1. Characteristics of patients who discontinued cBTKi and BCL2i therapy.
Table 3. Demographic and clinical characteristics of subgroups evaluated in the study.
Table 4. Real-world tumor response (rwR) for the immediate next line of therapya.
Figure 3. Time to real-world progression (rwP) or death (outcomes based on imaging, pathology, or laboratory data on left; all available evidence/methods of assessmenta on right). (a). cBTKi line of therapy. (b). BCL2i line of therapy. (c). Immediate next line of therapyb. aAll available evidence included all methods of assessment as observed in the patient record: imaging, pathology, laboratory report, constitutional symptoms, physical exam, or clinical assessment only. bOne patient was not evaluable in as the progression event occurred prior to the start of the immediate next line of therapy.
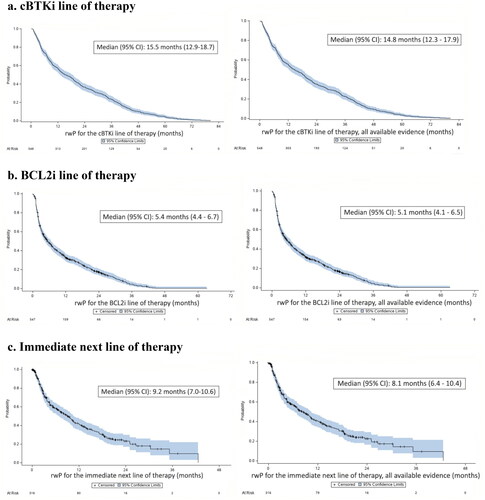
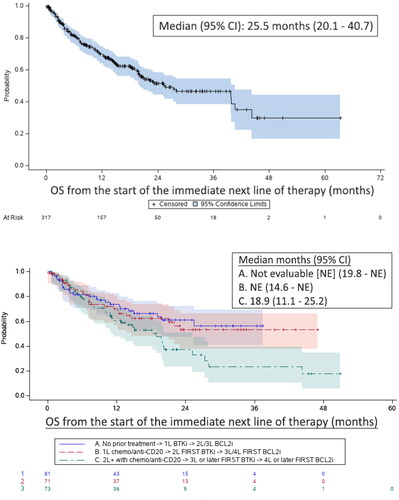
Figure 4. Overall survival (OS) from the start of the immediate next line of therapya (all patients above; subgroups by treatment historyb below). aThe immediate next line of therapy following the discontinuation of the initial cBTKi or initial BCL2i, whichever was later: bSubgroups included: (A) patients who initiated first-line therapy with a cBTKi and received BCL2i therapy in the second or third lines of treatment; (B) those who initiated the cBTKi in the second line of therapy, followed by BCL2i in the third or fourth lines; and (C) those who did not receive either a cBTKi until the third line or later and a BCL2i at the fourth line of therapy or later.