Figures & data
Table 1. Baseline demographics of patients with AML in remission post-HSCT.
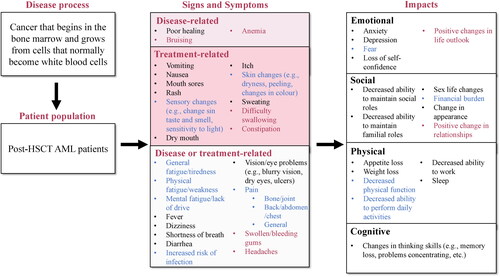
Figure 1. Conceptual model revised according to clinician interviews. Concepts included in preliminary conceptual model are in black text. Concepts added from clinician interviews are in red text. Concepts included in the preliminary conceptual model but revised following clinician interviews are in blue text. Symptoms were assigned to ‘disease-related’, ‘treatment-related’, or ‘disease or treatment-related’ based on their assignment in the preliminary conceptual disease model or based on answers given by clinicians during concept elicitation interviews. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant.
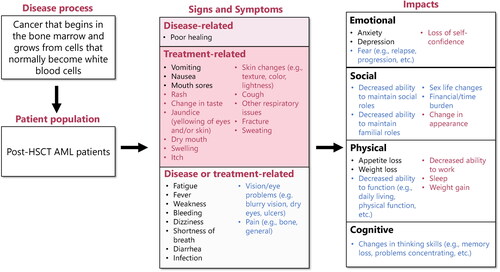
Figure 2. Frequency maps of symptoms mentioned by patients with AML in remission post-HSCT versus average peak bother rating: a) Total post-HSCT AML population; b) Maintenance therapy subpopulation. Bother was rated on a scale of 0–10, where 10 is the most severe. A symptom was deemed ‘salient’ if ≥50% of patients mentioned the symptom, either probed or spontaneous, and the average peak bother rating was ≥5. Some patients provided qualitative descriptions and would not provide a quantitative number, even after probing. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant
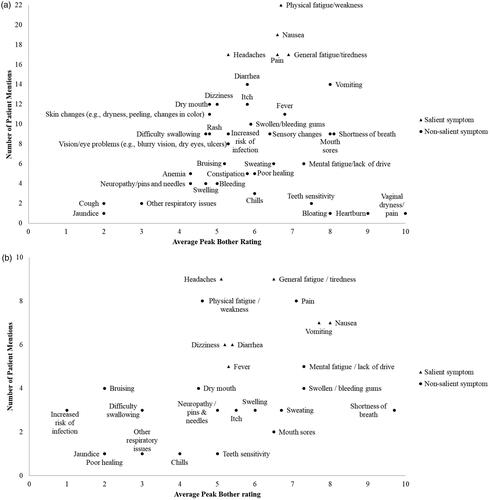
Figure 3. Frequency maps of impacts mentioned by patients with AML in remission post-HSCT versus average peak disturbance rating: a) Total post-HSCT AML population; b) Maintenance therapy subpopulation. Disturbance was rated on a scale of 0–10, where 10 is the most severe. An impact was deemed ‘salient’ if ≥50% of patients mentioned the impact, either probed or spontaneous, and the average peak disturbance rating was ≥5. Some patients provided qualitative descriptions and would not provide a quantitative number, even after probing. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant
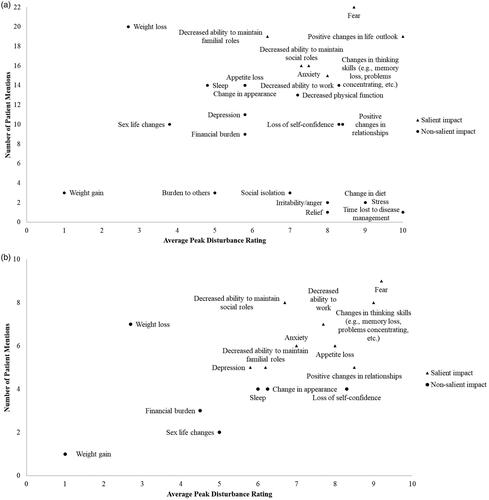
Figure 4. Conceptual model amended according to concept elicitation interviews with patients with AML in remission post-HSCT. Concepts included in the clinician-revised conceptual model are in black text. Concepts added from patient concept elicitation interviews are in red text. Concepts included in the clinician-revised conceptual model but amended following patient interviews are in blue text. Symptoms were assigned to ‘Disease-related’, ‘Treatment-related’, and ‘Disease or treatment-related’ based on their assignment in the preliminary conceptual model or based on answers given by clinicians or patients during concept elicitation interviews. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant.
Supplemental Material
Download MS Word (207.9 KB)Data availability statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
