Figures & data
Figure 1. Mean change in EORTC QLQ-C30 GHS/QoL (A), functioning (B), and symptoms (C) subscales, and EORTC QLQ-CLL16 subscales (D) from baseline to week 108 in all treated patients. Overall changes from baseline in a patient-reported EORTC QLQ-C30 (A–C) and EORTC QLQ-CLL16 (D) scores until week 108 of venetoclax treatment. (A) ≥5-point change indicated a clinically meaningful difference for the GHS/QoL subscale; error bars are 95% CI. (B, C, D) Clinical relevance of changes in HRQoL was determined based on the MID of values from baseline to each assessment time point. Five- to ten-point considered a little change while a ≥10-point change indicated a clinically meaningful difference. Improvement in functional subscales are indicated by positive change; improvement in symptom subscales are indicated by negative change. BL: baseline; CI: confidence interval; EORTC: European Organization for Research and Treatment of Cancer; QLQ-C30: Quality of Life Core Questionnaire Core 30; QLQ-CLL16: Quality of Life Questionnaire Chronic Lymphocytic Leukemia Module 16; GHS: global health status; HRQoL: health-related quality of life; MID: minimum important difference; OS: overall survival; PFS: progression-free survival; QoL: quality of life; Wk: week.
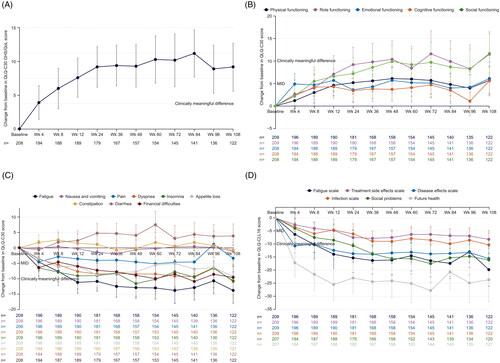
Table 1. Summary of any grade and grade ≥3 treatment emergent AEs occurring in ≥10% of patients and serious AEs occurring in ≥3% of patients treated with venetoclax.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select ‘Home’.
