Figures & data
Figure 1. Discontinuation status and timing of discontinuation among all elderly Medicare beneficiaries with CLL/SLL initiating ibrutinib.
Median (IQR) follow-up from ibrutinib initiation date was 2.1 (1.2, 3.3) years in the overall sample of 11,870 patients.
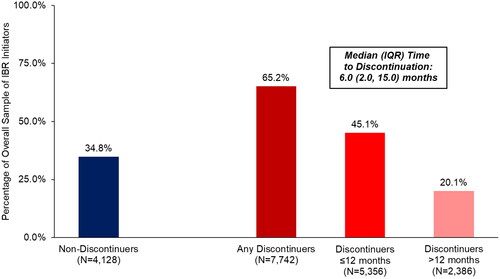
Table 1. Sample characteristics by discontinuation status among all elderly Medicare beneficiaries with CLL/SLL initiating ibrutinib.
Table 2. Cox regression results for factors associated with discontinuation among all elderly Medicare beneficiaries with CLL/SLL initiating ibrutinib.
Table 3. Initiation of another CLL/SLL treatment after discontinuation of ibrutinib among elderly Medicare beneficiaries with CLL/SLL by discontinuation status.
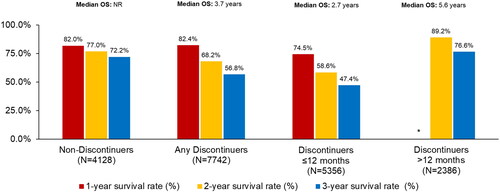
Figure 2. Overall survival from ibrutinib initiation among all elderly Medicare beneficiaries with CLL/SLL by discontinuation status.
*One-year OS rate not shown for discontinuers >12 months since by definition 100% of these patients would need to be alive for at least 12 months to qualify for inclusion in this group.
Appendix Table A1. ICD-9 and ICD-10 codes for other FDA-approved indications of ibrutinib.
Appendix Table A2. Sample attrition.
Appendix Table A3. Logistic regression results for discontinuation within 12 months (vs. disk >12 months) in the subset of patients who are discontinuers among Medicare beneficiaries with CLL/SLL initiating ibrutinib.
