Figures & data
Figure 1. Treatment schema. IV: intravenous; PO: orally. Ibrutinib was administered on days 1–28 of a 28-day cycle. Lenalidomide was administered on days 1–21 of a 28-day cycle. Dose-limiting toxicities were assessed during the first treatment cycle.
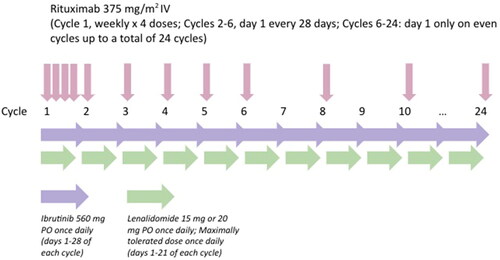
Table 1. Baseline characteristics.
Table 2. Grade ≥3 adverse events.
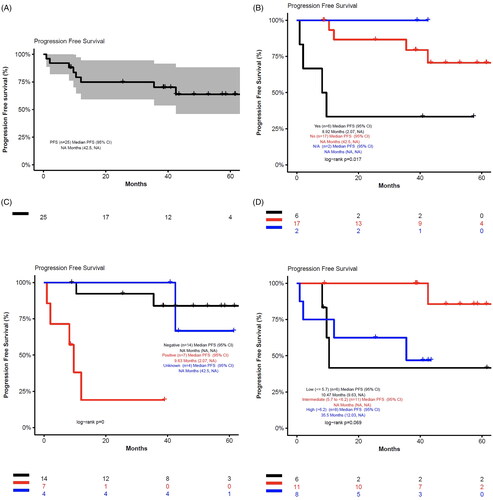
Figure 2. Best response in evaluable patients. Response was evaluated after cycles 2, 4, 6 and then every 3 cycles thereafter until disease progression or discontinuation of study using Cheson criteria 2007. Best overall response was defined as the percentage of patients who achieved a CR or PR. Clinical benefit rate was defined as the percentage of patients who achieved CR, PR, or SD for at least 2 months. One patient who was not evaluable for response was excluded.
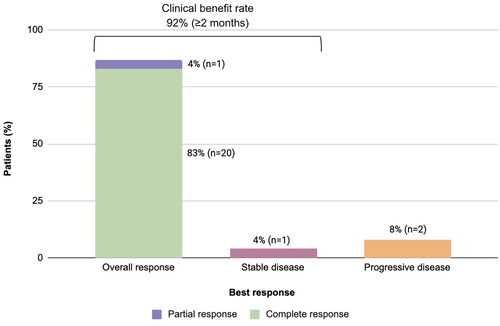
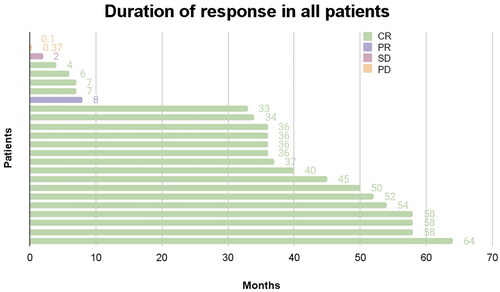
Figure 4. Duration of response. CR: complete response; PR: partial response; SD: Stable disease; PD: progressive disease. Duration of response in 24 evaluable patients. Duration of response was defined as the number of months from first documented response to the date of first event (disease progression or death) or censoring prior to the data cutoff. One patient who was not evaluable for response was excluded.
Supplemental Material
Download MS Word (237.7 KB)Data availability statement
The data that support the findings of this study are available on request from the corresponding author, AI. The data are not publicly available due to their containing information that could compromise the privacy of research participants. The study involves a review of the electronic medical records of mantle cell lymphoma patients who received R2I. The research data set has removed most, but not all protected health information (for example: actual dates of response assessments and treatment were included as needed for analysis but would be considered PHI). The dataset is also considered property of Hackensack Meridian Health and not owned by the investigators. Therefore, for both reasons, the dataset cannot be made openly available. However, upon request we are willing to share portions of the data for appropriate review. Requests can be made through the corresponding author or directly to representatives of Hackensack Meridian Health (Dr. Andrew Ip; Email: [email protected]).